Standard Gibbs Free Energy Equation
The standard gibbs free energy of formation of an element in its standard state is 0 δg ƒ element 0 values of standard gibbs free energy of formation can be used to calculate the change in gibbs free energy for a chemical reaction or physical change. A b c d.
 Calculate The Standard Change In Gibbs Free Energy Fo
Calculate The Standard Change In Gibbs Free Energy Fo
Its symbol is δ f g.
Standard gibbs free energy equation. The standard gibbs free energy of formation of a compound is the change of gibbs free energy that accompanies the formation of 1 mole of that substance from its component elements at their standard states the most stable form of the element at 25 c and 100 kpa. The gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. G h ts the gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions.
Gibbs free energy equation. Gibbs free energy g the energy associated with a chemical reaction that can be used to do work. The units of δg if you look up or calculate the value of the standard free energy of a reaction you will end up with units of kj mol 1 but if you look at the units on the right hand side of the equation they include j not kj.
The free energy of a system is the sum of its enthalpy h plus the product of the temperature kelvin and the entropy s of the system. Using standard change in gibbs free energy δ g the change in gibbs free energy under nonstandard conditions δ g can be determined from the standard change in gibbs free energy δ g. Or the total change in any of the property is zero.
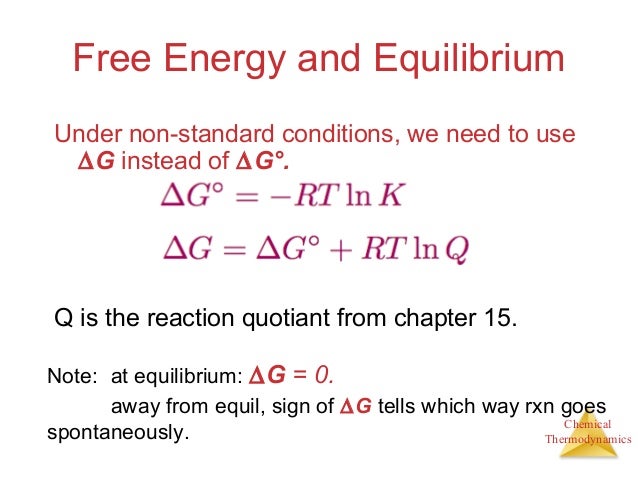
Looking at the following equation we can say if the reaction is reversible and the gibbs free energy is zero then the system is said to be in equilibrium. δ g δ g rt ln q where r is the ideal gas constant 8 314 j mol k q is the reaction quotient and t is the temperature in kelvin. Gibbs free energy denoted g combines enthalpy and entropy into a single value.
Triangle r g 0. When a system changes from an initial state to a final state the gibbs free energy δg equals the work exchanged by the system with its surroundings minus the work of the pressure force. Free energy of reaction g.
You must convert your standard free energy value into joules by multiplying the kj value by 1000. The change in free energy δ g is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. The gibbs free energy equation is dependent on pressure.
δ g can predict the direction of the chemical reaction under two conditions.
 Solved Calculate The Equilibrium Constant From The Standa
Solved Calculate The Equilibrium Constant From The Standa
 Calculate The Standard Change In Gibbs Free Energy For The
Calculate The Standard Change In Gibbs Free Energy For The
 17 1 Equilibrium And Gibbs Free Energy Hl Youtube
17 1 Equilibrium And Gibbs Free Energy Hl Youtube
 17 1 Equilibrium And Gibbs Free Energy Hl Youtube
17 1 Equilibrium And Gibbs Free Energy Hl Youtube
 Gibbs Free Energy Example Video Khan Academy
Gibbs Free Energy Example Video Khan Academy
Department Of Chemistry And Biochemistry Chem 4520 Metabolic
 Standard Gibbs Free Energy Change And Standard Enthtlyy Changes Of
Standard Gibbs Free Energy Change And Standard Enthtlyy Changes Of
 Ppt Gibbs Free Energy Powerpoint Presentation Free Download
Ppt Gibbs Free Energy Powerpoint Presentation Free Download
Department Of Chemistry And Biochemistry Chem 4520 Metabolic
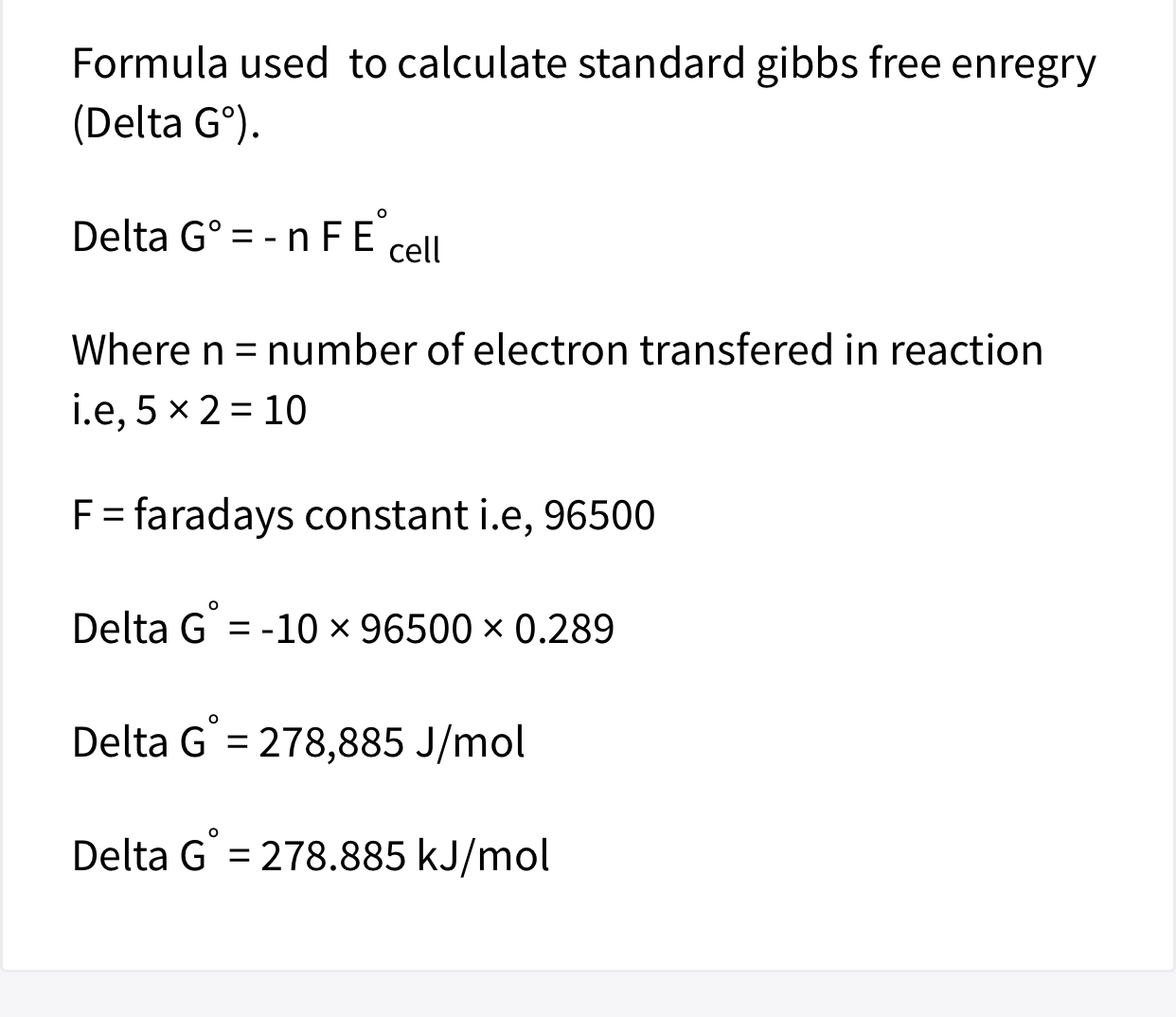 Answered 6 What Is The Standard Gibbs Free Bartleby
Answered 6 What Is The Standard Gibbs Free Bartleby
Gibbs Energy And Reaction Diagrams Chemical Kinetics Flashcards
Gibbs Free Energy Equilibrium Constant Mastering Chemistry
 Getting Gibbs Energy As A Function Of Temperature
Getting Gibbs Energy As A Function Of Temperature
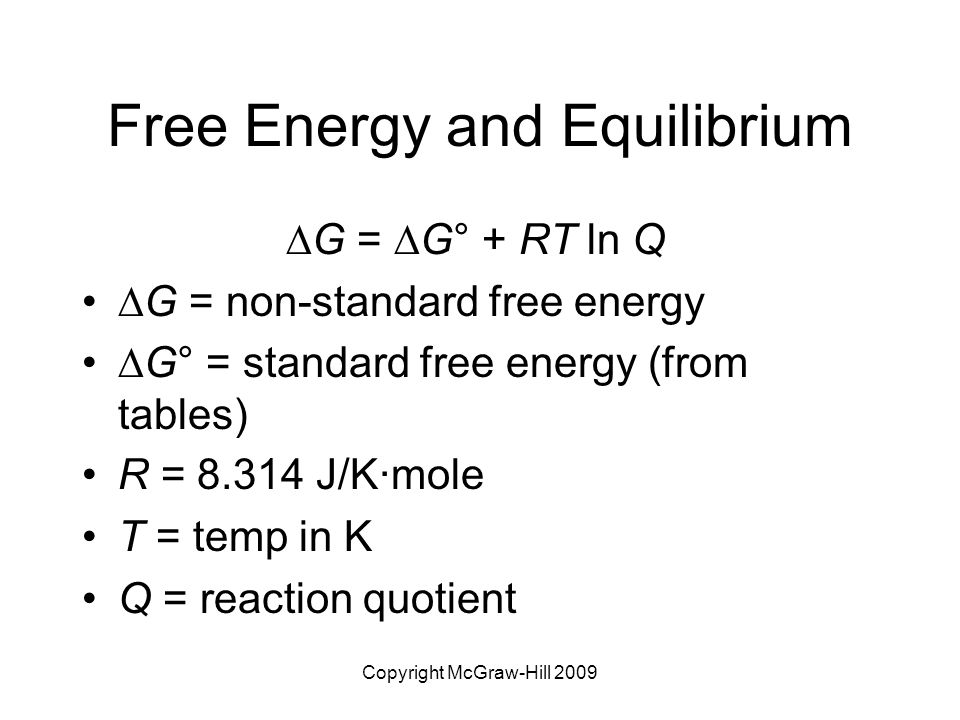 Chapter 18 Entropy Free Energy And Equilibrium Ppt Video Online
Chapter 18 Entropy Free Energy And Equilibrium Ppt Video Online
 Chem 2 Std Free Energy Of Formation Vii
Chem 2 Std Free Energy Of Formation Vii
 Stoichiometric Equations And Standard Gibbs Free Energy Changes
Stoichiometric Equations And Standard Gibbs Free Energy Changes
 Calculate The Standard Change In Gibbs Fre Clutch Prep
Calculate The Standard Change In Gibbs Fre Clutch Prep
How Is Gibbs Free Energy Related To Enthalpy And Entropy Socratic
 Ap Chemistry Non Standard Gibbs Free Energy Worksheet Review
Ap Chemistry Non Standard Gibbs Free Energy Worksheet Review
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsox Hhyvknjanf1tnseoz72om4nntckufqvm Ohk5acd Nmhoz Usqp Cau
 Reaction Quotient And Gibbs Free Energy At The Start Of A Reaction
Reaction Quotient And Gibbs Free Energy At The Start Of A Reaction
 Standard Cell Potential And Free Energy Worked Example Youtube
Standard Cell Potential And Free Energy Worked Example Youtube
 15 2 Gibbs Free Energy Hl Youtube
15 2 Gibbs Free Energy Hl Youtube
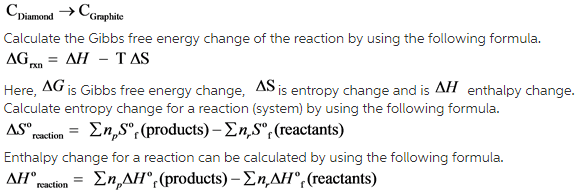 What Is The Standard Gibbs Free Energy For The Transformation Of
What Is The Standard Gibbs Free Energy For The Transformation Of
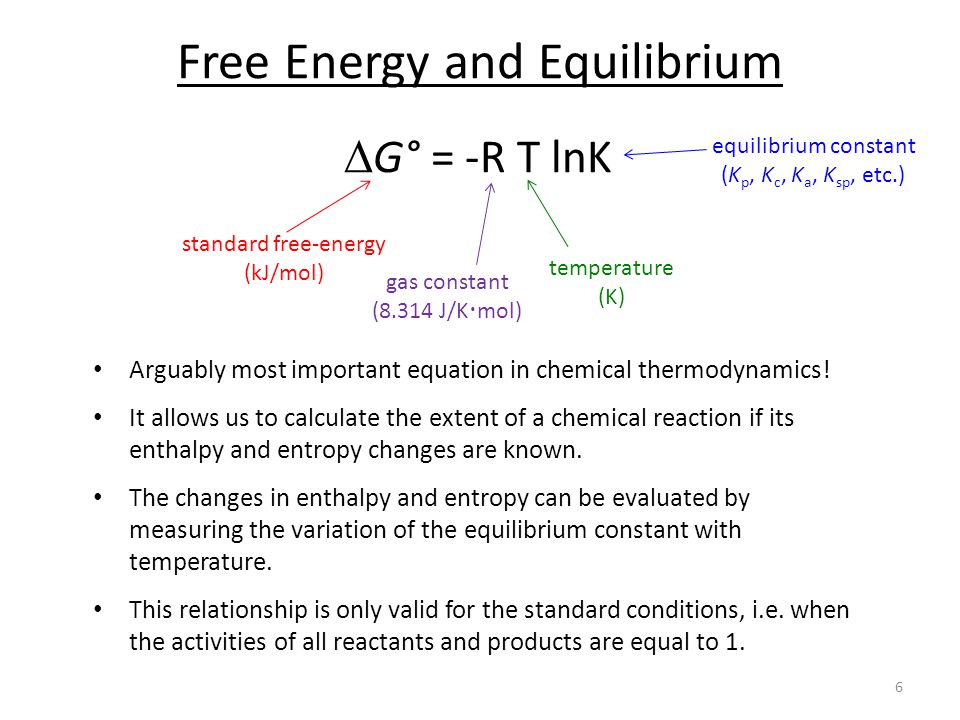 Calculate Standard Gibbs Free Energy Energy Etfs
Calculate Standard Gibbs Free Energy Energy Etfs
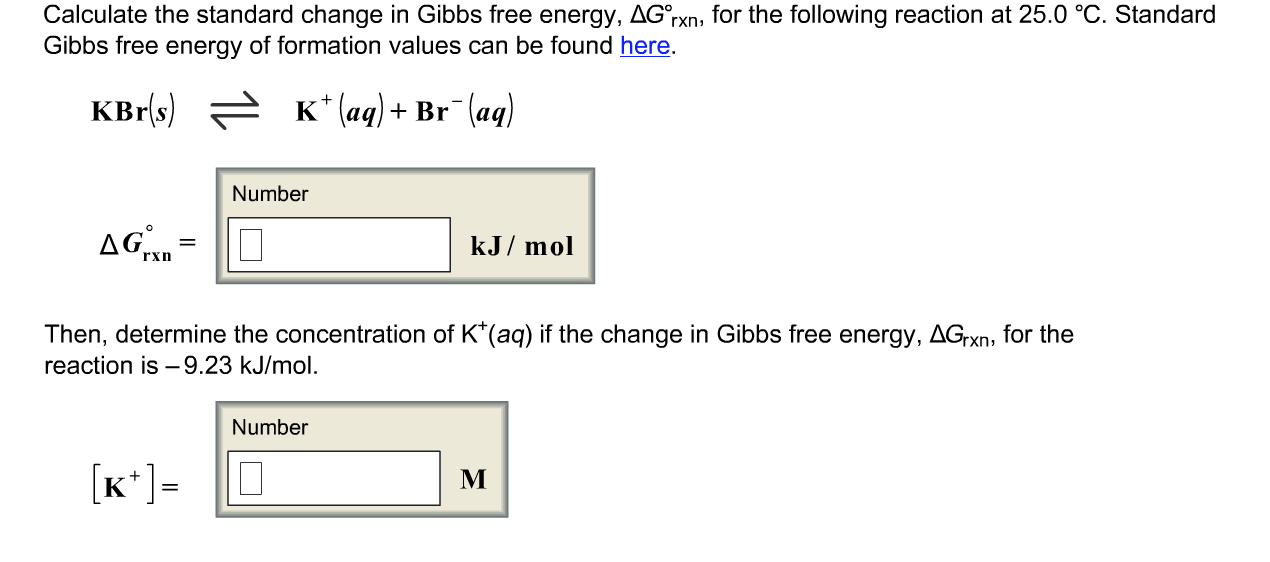 Solved Calculate The Standard Change In Gibbs Free Energy
Solved Calculate The Standard Change In Gibbs Free Energy
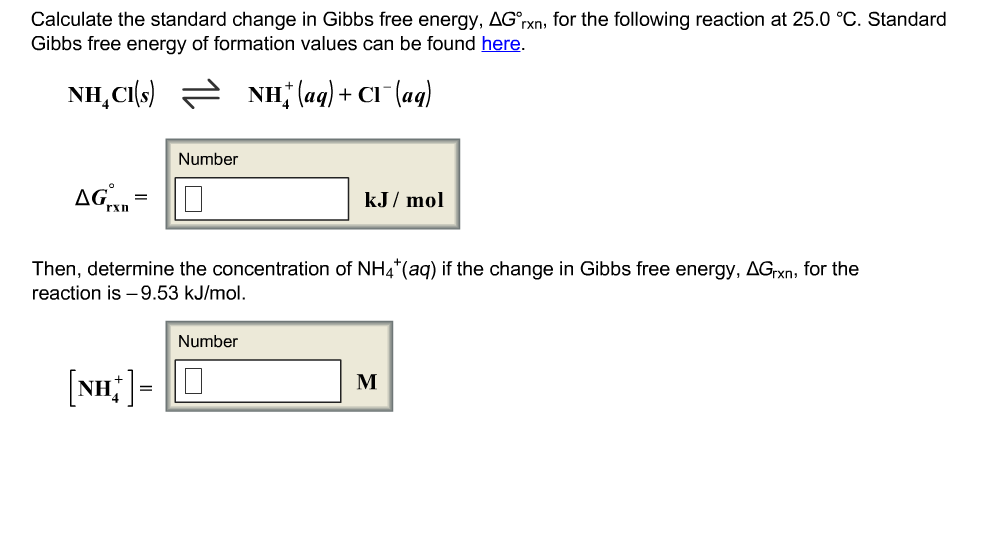 Solved Calculate The Standard Change In Gibbs Free Energy
Solved Calculate The Standard Change In Gibbs Free Energy
 Solved Calculate The Standard Change In Gibbs Free Energy
Solved Calculate The Standard Change In Gibbs Free Energy
Helmholtz And Gibbs Free Energies
 Temperature In The Gibbs Free Energy Equation Chemistry Stack
Temperature In The Gibbs Free Energy Equation Chemistry Stack
Free Energy Changes In Biochemical Systems
 Temperature In The Gibbs Free Energy Equation Chemistry Stack
Temperature In The Gibbs Free Energy Equation Chemistry Stack
 Chemical Thermodynamics Chapter 17 Chemical Thermodynamics Ppt
Chemical Thermodynamics Chapter 17 Chemical Thermodynamics Ppt
Gibbs Free Energy And The Spontaneity Of Chemical Reactions

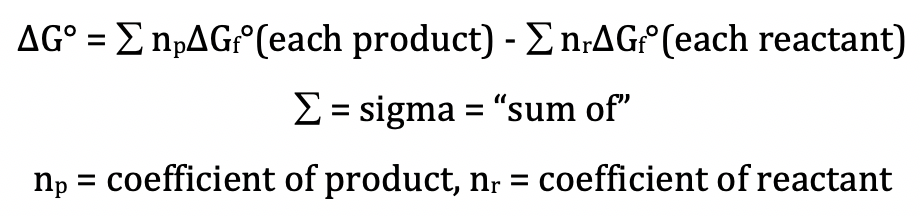

Posting Komentar
Posting Komentar