Ionization Energy Atomic Radius Periodic Trends
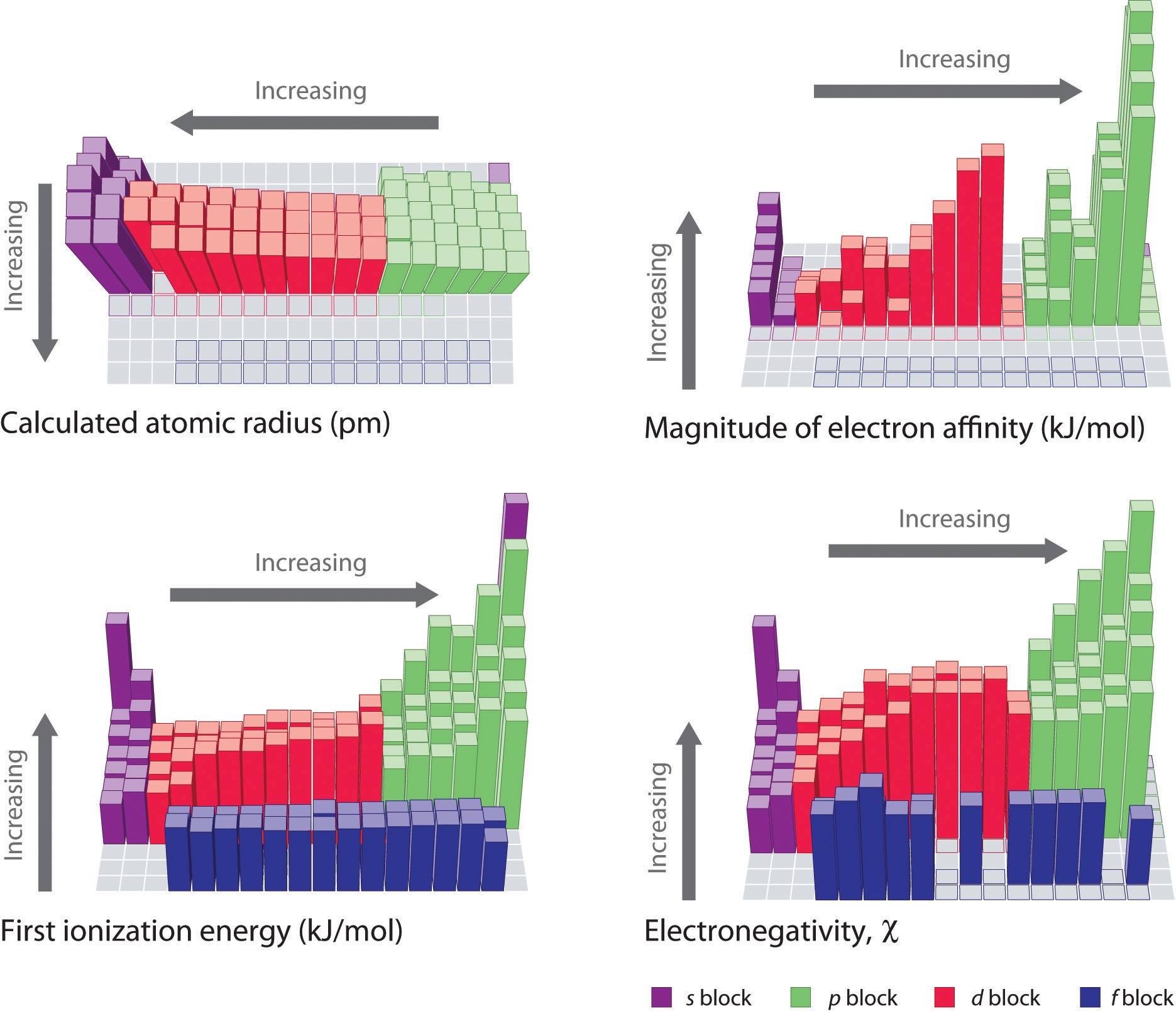
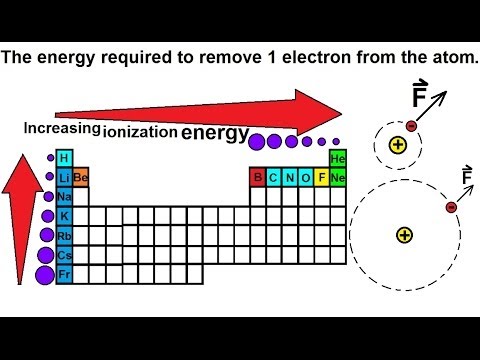
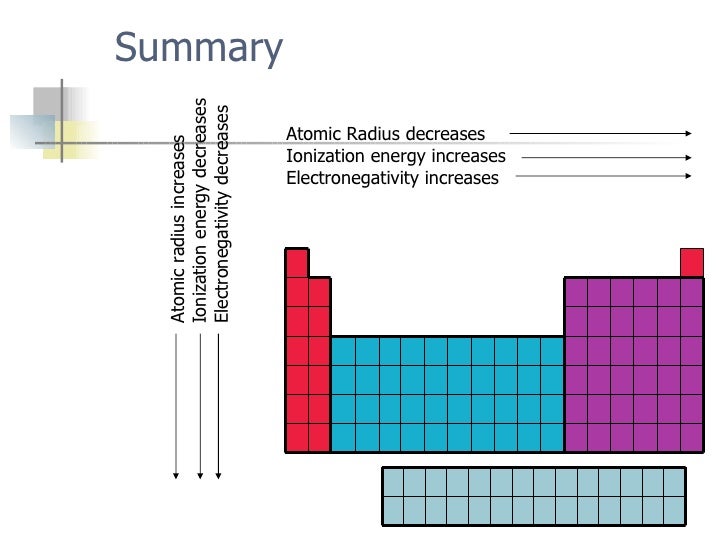
Thus the ionization energy decreases as well. As you go from left to right you go from low ionization energy to high ionization energy.
 Easy To Use Chart Of Periodic Table Trends Science Notes
Easy To Use Chart Of Periodic Table Trends Science Notes
As radius increases the electrostatic force decreases.
Ionization energy atomic radius periodic trends. By choosing elements from the periodic table atoms can be selected for a side by side comparison and analysis. When you go down a group there is a decline in the ionization energy. Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element including its size and its electronic properties.
Ionization energy increases as you move across a period left to right because the increasing number of protons attracts the electrons more strongly making it harder to remove one. Ionization energy is the smallest amount of energy needed to pull an electron away from an atom in the gas state. So this is high high ionization energy and that s the general trend across the periodic table.
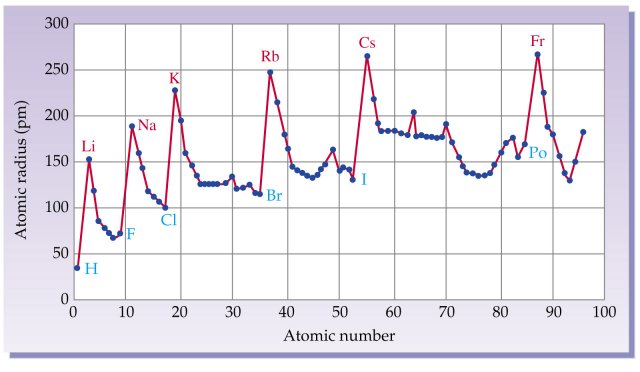
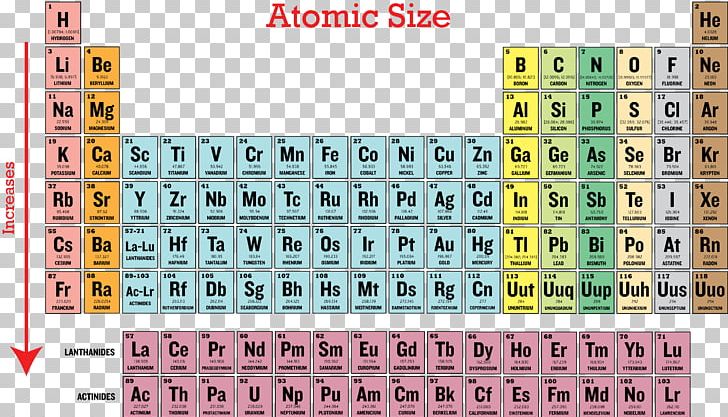
The atomic radius for atoms of an element tends to go up as you move down a group of elements in the table. This is because the number of protons increases in this direction and these are able to pull the electrons strongly. Ionization energy sees a rise when you move from left to right across a period.
It is important to know that an atom has as many ionization energies as it has electrons and the second or third ionization energy is always bigger than the second or first respectively. Students can also attempt to ionize an atom by removing its valence electrons. Electronegativity ionization energy electron affinity atomic radius melting point and metallic character.
The atomic radius increases as you move down a column because for every new row of the table a new electron shell is added to the atom. 50 videos play all mix periodic trends atomic radius ionization energy youtube intro to chemistry basic concepts periodic table elements metric system unit conversion duration. In addition noble gases have the highest ionization energies due to their completed outer shells.
Periodic trends atomic size ionization energy and metallic character chemistry libretexts. Certain properties notably atomic radius ionization energy electron affinity and metallic character can be qualitatively understood by the positions of the elements on the periodic table. While the atomic radius can be defined in a number of different ways the general atomic radius trend across the periodic table holds true.
Major periodic trends include. In this simulation students can investigate the periodic trends of atomic radius ionization energy and ionic radius.
 8 7 Periodic Properties Of The Elements Chemistry Libretexts
8 7 Periodic Properties Of The Elements Chemistry Libretexts
 Periodic Properties Of Elements With Examples Online Chemistry
Periodic Properties Of Elements With Examples Online Chemistry
 Periodic Trends Electronegativity Ionization Energy And Atomic
Periodic Trends Electronegativity Ionization Energy And Atomic
:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png) Easy To Use Chart Of Periodic Table Trends
Easy To Use Chart Of Periodic Table Trends
 The Octet Rule And Periodic Trends The Cavalcade O Chemistry
The Octet Rule And Periodic Trends The Cavalcade O Chemistry
Periodic Trends Presentation Chemistry
 Atomic Radii And Periodic Properties
Atomic Radii And Periodic Properties
 Chapter 8 Br Section E Br Periodic Trends
Chapter 8 Br Section E Br Periodic Trends
 Ionization Energy Periodic Table Periodic Trends Atomic Radius
Ionization Energy Periodic Table Periodic Trends Atomic Radius
Trends In The Periodic Table Course Hero
 Periodic Trends Atomic Radius Ionization Energy Electronegativity
Periodic Trends Atomic Radius Ionization Energy Electronegativity
Periodic Table Trends Electronegativity Atomic Radius
Ionization Energy And Electronegativity
 Chemistry Periodic Variations 13 Of 23 Atomic Radius
Chemistry Periodic Variations 13 Of 23 Atomic Radius
 Periodic Trends We Will Explain Observed Trends In Atomic And
Periodic Trends We Will Explain Observed Trends In Atomic And
 Periodic Table Of Elements Periodic Trends Periodic Table Valence
Periodic Table Of Elements Periodic Trends Periodic Table Valence
 Ionization Energy Periodic Table Periodic Trends Atomic Radius Png
Ionization Energy Periodic Table Periodic Trends Atomic Radius Png
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png) Easy To Use Chart Of Periodic Table Trends
Easy To Use Chart Of Periodic Table Trends
 Atomic Radius Trends On Periodic Table Video Khan Academy
Atomic Radius Trends On Periodic Table Video Khan Academy
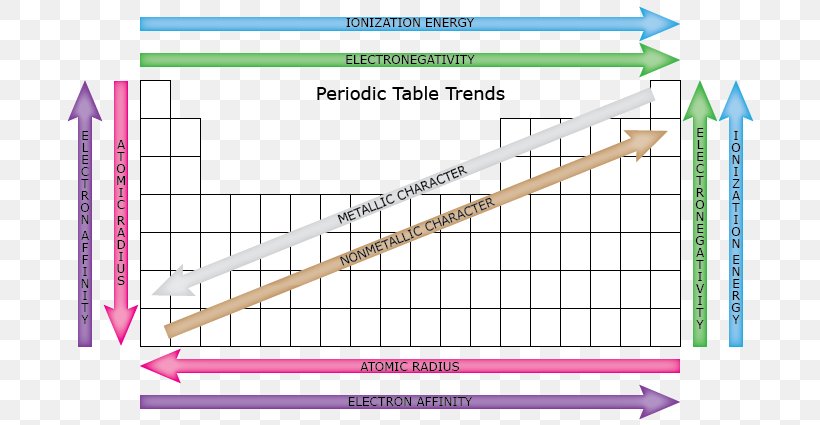 Periodic Trends Periodic Table Atomic Radius Electronegativity
Periodic Trends Periodic Table Atomic Radius Electronegativity
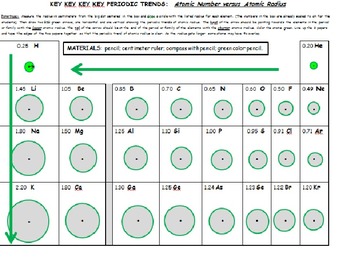 Periodic Trends Electronegativity Ionization Energy And Atomic
Periodic Trends Electronegativity Ionization Energy And Atomic
 Periodic Trends Ppt Video Online Download
Periodic Trends Ppt Video Online Download
 Periodic Table Atomic Radius Ionization Energy Electronegativity
Periodic Table Atomic Radius Ionization Energy Electronegativity
 Chart Of Periodic Table Trends Science Notes Chemistry Lessons
Chart Of Periodic Table Trends Science Notes Chemistry Lessons
What Are The Periodic Trends For Atomic Radii Ionization Energy
 Periodic Trends Periodic Table Atomic Radius Electronegativity
Periodic Trends Periodic Table Atomic Radius Electronegativity
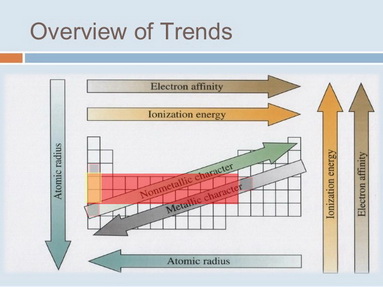 If Element X Has An Atomic Radius Larger Than Ga An Electron
If Element X Has An Atomic Radius Larger Than Ga An Electron
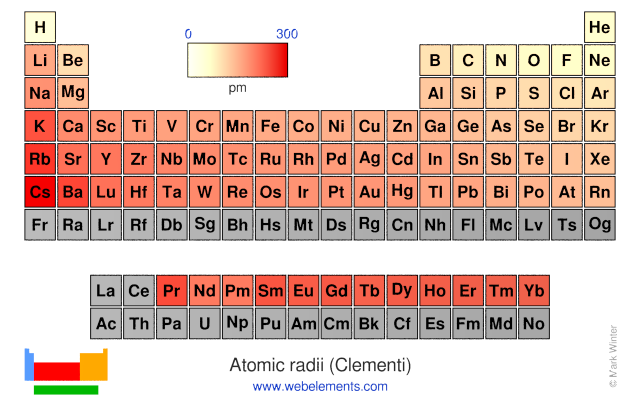 Webelements Periodic Table Periodicity Atomic Radii Clementi
Webelements Periodic Table Periodicity Atomic Radii Clementi
Jeeyoon S Chemistry Blog Periodic Trends
 Periodic Trends Periodic Table Atomic Radius Electronegativity
Periodic Trends Periodic Table Atomic Radius Electronegativity
 Periodic Trends Electronegativity Ionization Energy And Atomic
Periodic Trends Electronegativity Ionization Energy And Atomic
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcro0crxtml5y8c Lmixwqvkayt8b3zydgpnaxbzkp7ub8tyzuo9 Usqp Cau
 Overview Atomic Size Ionization Energy Electron Affinity Youtube
Overview Atomic Size Ionization Energy Electron Affinity Youtube
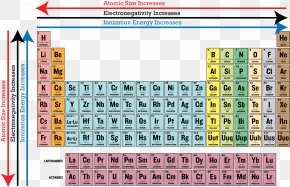 Ionization Energy Periodic Table Periodic Trends Atomic Radius
Ionization Energy Periodic Table Periodic Trends Atomic Radius



Posting Komentar
Posting Komentar