Standard Gibbs Free Energy Of Formation Table
δ g 90 46 32 01 k j m o l 58 45 k j m o l. The standard enthalpy of formation δh 0 f of a compound is the change in enthalpy that accompanies the formation of 1 mole of a compound from its elements with all substances in their standard states.
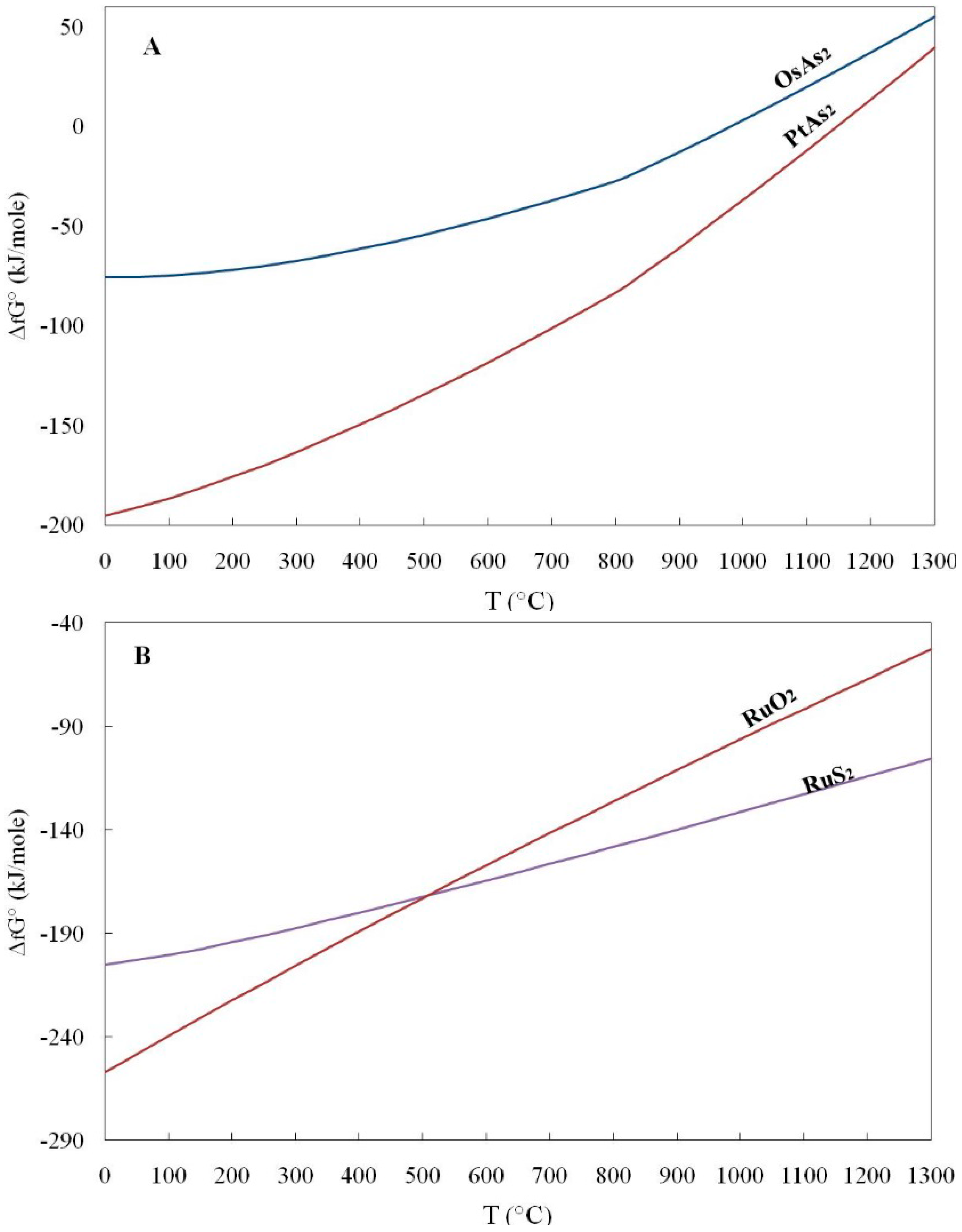 Geosciences Free Full Text Gibbs Free Energy Of Formation For
Geosciences Free Full Text Gibbs Free Energy Of Formation For
Chemical substance state g f kj mol.

Standard gibbs free energy of formation table. Gibbs free energy denoted g combines enthalpy and entropy into a single value. º gibbs free energy is a state function just as enthalpy and entropy are. So we could write a chemical equation to represent the formation of each compound as shown below.
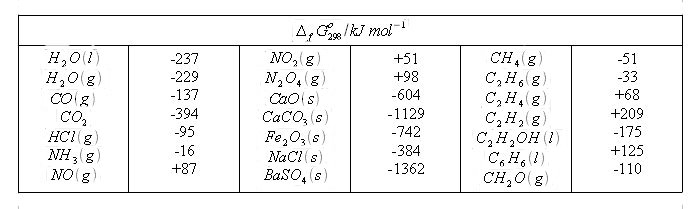
Chem table enthalpy of formation. This means that the d g of the sum of a series of reactions is equal to the sum of the d g s of the individual reactions. δg ƒ hcl g 95 3 kj mol 1.
The standard gibbs free energy of formation at 25 c 298 15 k for 1 mol of the substance in its given state g gas and l liquide from its elements in their standard state stable forms at 1 bar and 25 c s. Standard free energy of formation d g f º standard free energy of formation. These values are valid for the temperature 25 c.
Using the table of values of standard gibbs free energy of formation δg ƒ above we find that. The standard gibbs free energy of formation of a compound is the change of gibbs free energy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states the most stable form of the element at 1 bar of pressure and the specified temperature usually 298 15 k or 25 c. δ g can predict the direction of the chemical reaction under two conditions.
The change in free energy δ g is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system. Ag aq 78. Calculate δ h delta h demonstrated example 1 chem table gibbs free energy of formation delta g may 2nd 2010 author.
δg ƒ nh 4 cl s 202 7 kj mol 1. Both ways to calculate the standard free energy change at 25 c give the same numerical value to three significant figures and both predict that the process is nonspontaneous not spontaneous at room temperature since δ g o 0. δg ƒ nh 3 g 16 5 kj mol 1.
The table below shows the standard enthalpy of formation the standard gibbs free energy of formation standard entropy and molar heat capacity at constant pressure of several inorganic compounds. The standard entropy for 1 mol of the substance in its given state g gas and l liquide at 1 bar and 25 c. Standard heats and free energies of formation and absolute entropies of elements and inorganic compounds.
Appendix 4 standard gibbs energies and enthalpies of formation at 298 k 1 atm ph 7 and 0 25 m ionic strength substance δgo kj mol δho kj mol atp 2097 89 2995 59 adp 1230 12 2005 92 amp 360 29 1016 88 adenosine 529 96 5 34 p.
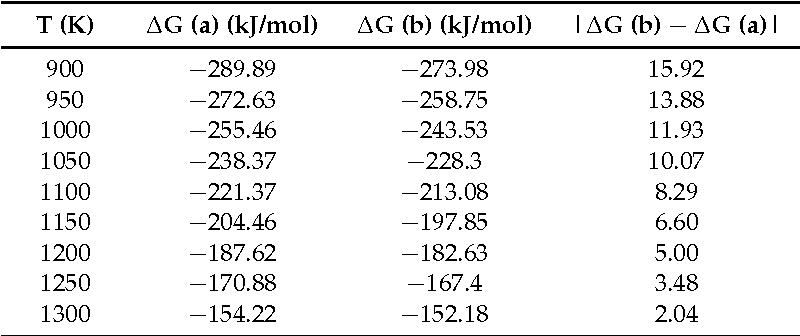 Table 1 From Gibbs Free Energy Of Formation For Selected Platinum
Table 1 From Gibbs Free Energy Of Formation For Selected Platinum
 Appendix C Standard Enthalpy And Gibbs Free Energy Of Reaction
Appendix C Standard Enthalpy And Gibbs Free Energy Of Reaction
 Standard Free Energies Of Formation Kj Mol For Selected Binary
Standard Free Energies Of Formation Kj Mol For Selected Binary
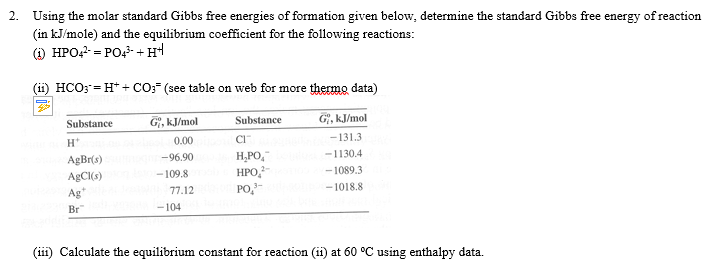 Solved Using The Molar Standard Gibbs Free Energies Of Fo
Solved Using The Molar Standard Gibbs Free Energies Of Fo
 Standard Gibbs Free Energy Of Formation Standard Enthalpy Of
Standard Gibbs Free Energy Of Formation Standard Enthalpy Of
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcszdyfosgm8dz1nrdz2fv Reaeclgxzjttvyrq0ny4amqgmp Lk Usqp Cau
 2012 General Chemistry I 1 Chapter 8 Thermodynamics The Second
2012 General Chemistry I 1 Chapter 8 Thermodynamics The Second
File Standard Free Energies Of Formation Jpg Chemprime
Http Employees Oneonta Edu Viningwj Chem112 Chapter 19 Thermo Full Pdf
 Standard Gibbs Free Energy Of Formation Standard Enthalpy Of
Standard Gibbs Free Energy Of Formation Standard Enthalpy Of
 Table 1 From Estimates Of Gibbs Free Energies Of Formation Of
Table 1 From Estimates Of Gibbs Free Energies Of Formation Of
 Table 5 From Standard Gibbs Free Energies Of Reactions Of Ozone
Table 5 From Standard Gibbs Free Energies Of Reactions Of Ozone
 Gibbs Free Energy Change An Overview Sciencedirect Topics
Gibbs Free Energy Change An Overview Sciencedirect Topics
 Standard Gibbs Free Energy Of Formation Of Aqueous Salts
Standard Gibbs Free Energy Of Formation Of Aqueous Salts
Human Free Energy Table Hmolpedia
 The Standard Gibbs Free Energy Of Formation Of Is Zero Energy Etfs
The Standard Gibbs Free Energy Of Formation Of Is Zero Energy Etfs
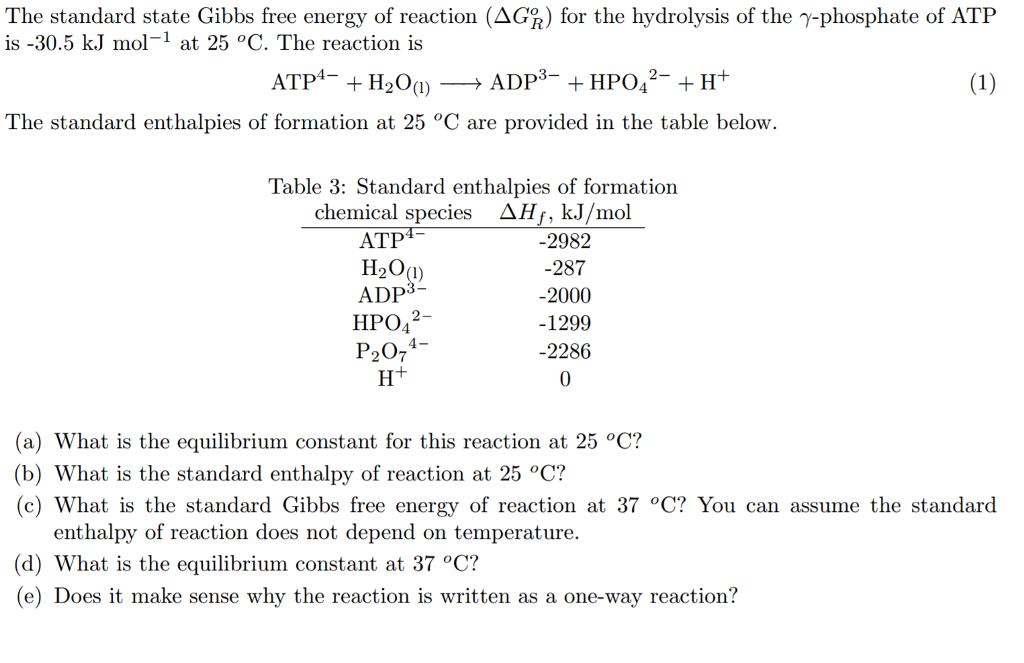 Solved The Standard State Gibbs Free Energy Of Reaction
Solved The Standard State Gibbs Free Energy Of Reaction
 Gibbs Free Energy Of Formation An Overview Sciencedirect Topics
Gibbs Free Energy Of Formation An Overview Sciencedirect Topics
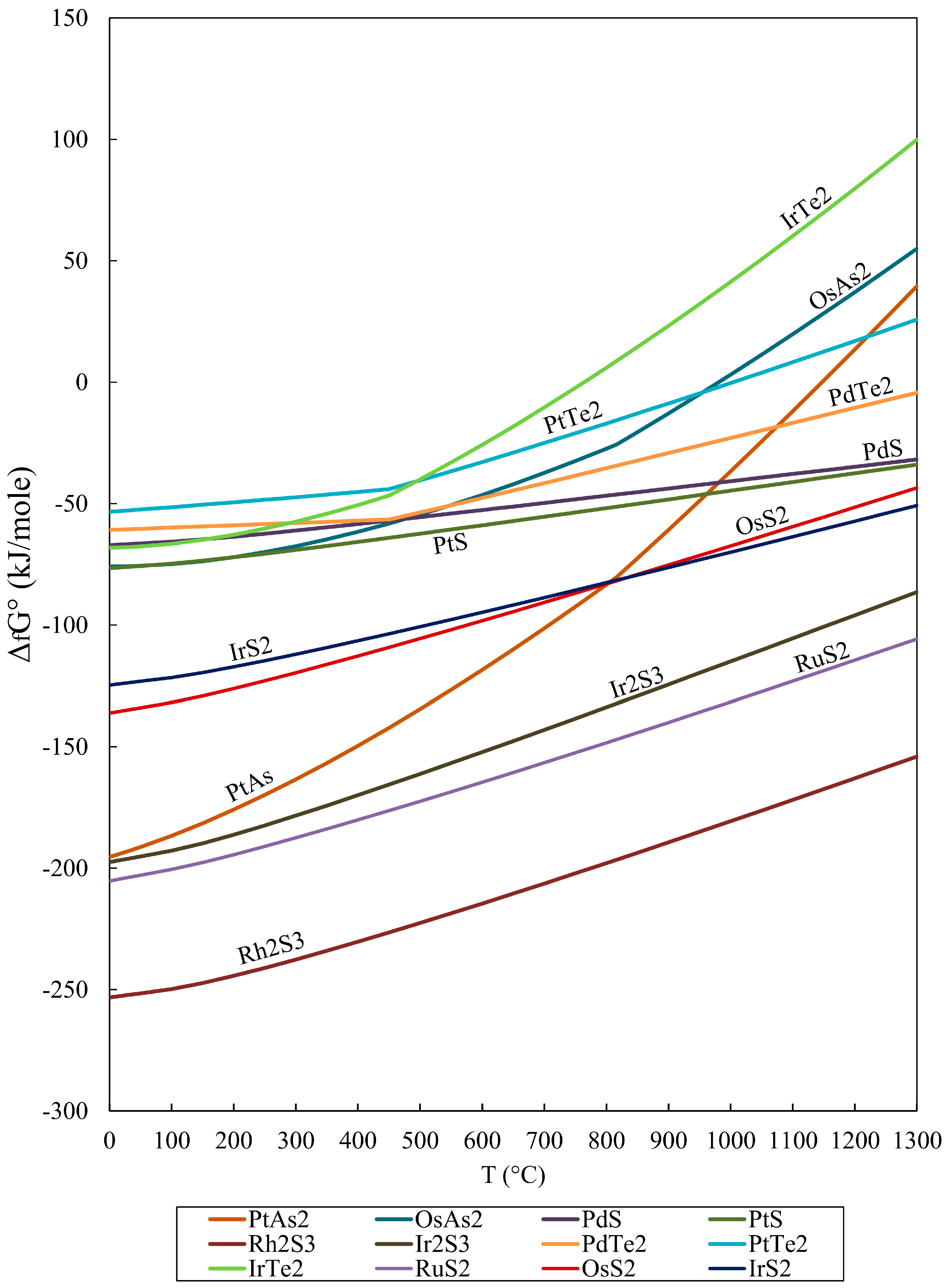 Geosciences Free Full Text Gibbs Free Energy Of Formation For
Geosciences Free Full Text Gibbs Free Energy Of Formation For
 Thermodynamic Study Of Iron In Alloys Of Quasicrystalline Alcufe
Thermodynamic Study Of Iron In Alloys Of Quasicrystalline Alcufe
 Table 1 From Gibbs Free Energy Of Formation For Selected Platinum
Table 1 From Gibbs Free Energy Of Formation For Selected Platinum
 Standard Free Energy Changes Introduction To Chemistry
Standard Free Energy Changes Introduction To Chemistry
 Calculate The Standard Change In Gibbs Free Energy For The
Calculate The Standard Change In Gibbs Free Energy For The
 Models For The Estimation Of Thermodynamic Properties Of Layered
Models For The Estimation Of Thermodynamic Properties Of Layered
 Standard Gibbs Free Energies Of Formation Of Lead And Related
Standard Gibbs Free Energies Of Formation Of Lead And Related
Http Www Soest Hawaii Edu Oceanography Glazer Pdfs Courses Ocn623 Lectures 03 Thermodynamics Pdf
 Standard Enthalpy Of Formation And Standard Free Energy Of
Standard Enthalpy Of Formation And Standard Free Energy Of
 Freeenergylesson2 Wikieducator
Freeenergylesson2 Wikieducator
 Gibbs Free Energy Of Formation For Mno Al2o3 Download Table
Gibbs Free Energy Of Formation For Mno Al2o3 Download Table
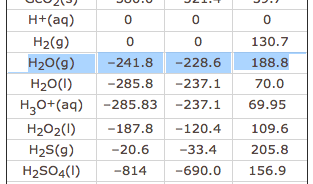 Solved Calculate The Standard Change In Gibbs Free Energy
Solved Calculate The Standard Change In Gibbs Free Energy
 Table 4 From Gibbs Free Energy Of Formation For Selected Platinum
Table 4 From Gibbs Free Energy Of Formation For Selected Platinum
Http Www Soest Hawaii Edu Oceanography Courses Ocn623 Spring2013 Chemical Equilibrium 2013 Handouts Pdf
 Models For The Estimation Of Thermodynamic Properties Of Layered
Models For The Estimation Of Thermodynamic Properties Of Layered
 Appendix C Standard Enthalpy And Gibbs Free Energy Of Reaction
Appendix C Standard Enthalpy And Gibbs Free Energy Of Reaction
 Review Of The Synthesis Of Layered Double Hydroxides A
Review Of The Synthesis Of Layered Double Hydroxides A
 Chem 2 Std Free Energy Of Formation Vii
Chem 2 Std Free Energy Of Formation Vii


Posting Komentar
Posting Komentar