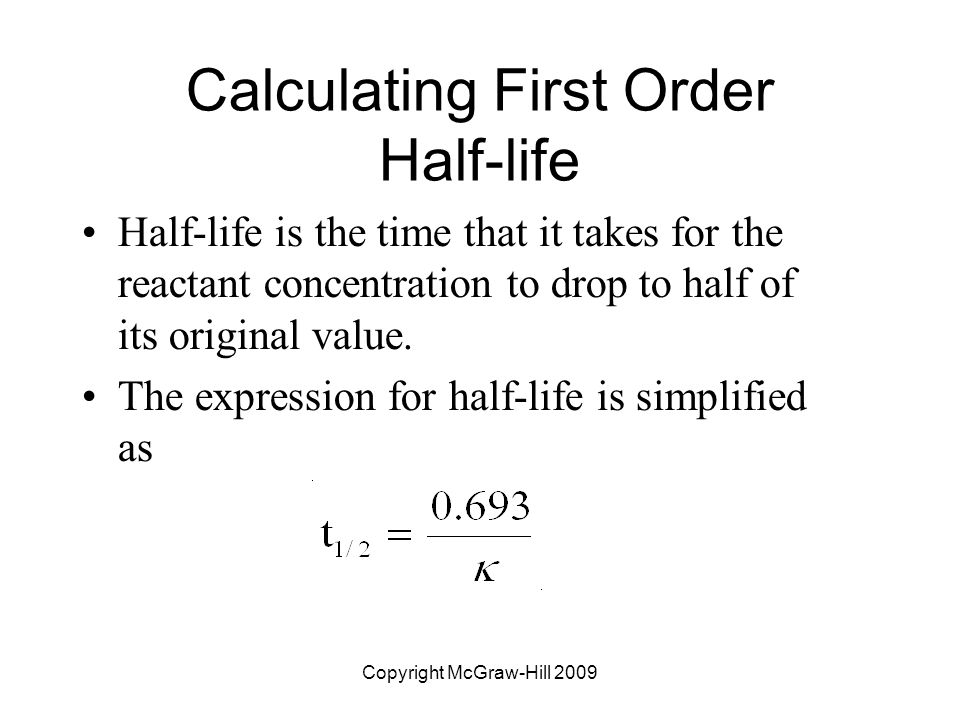
Half Life Equation First Order
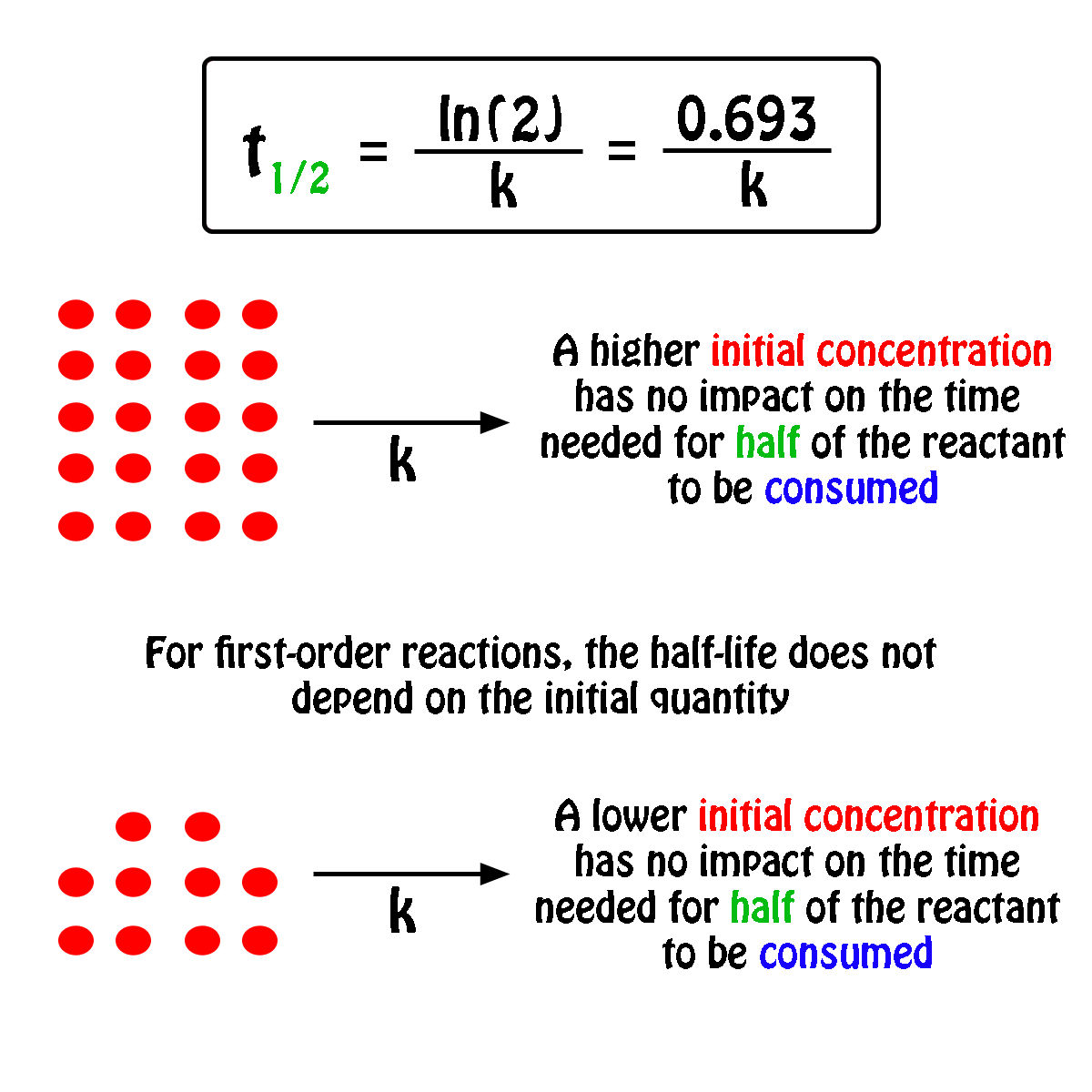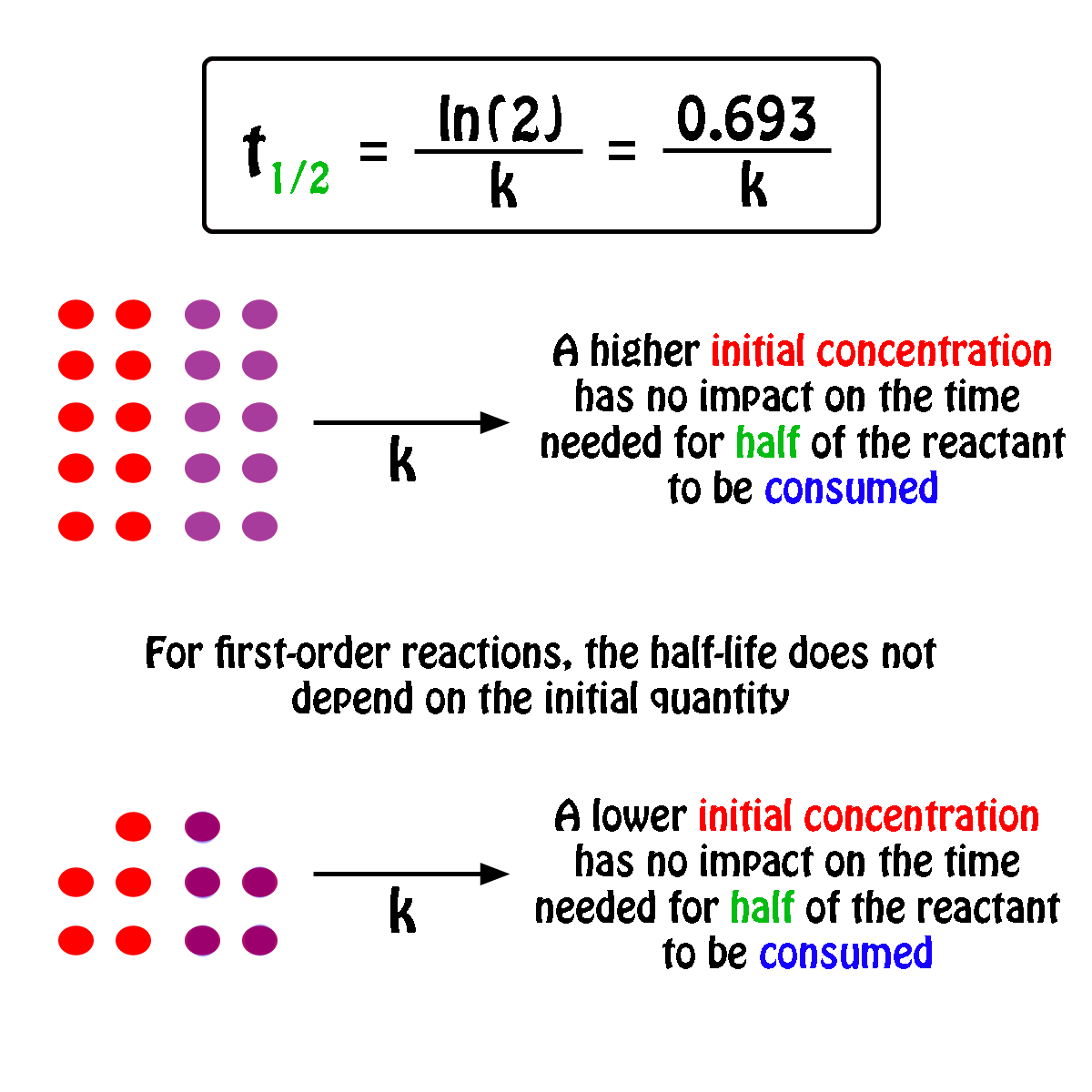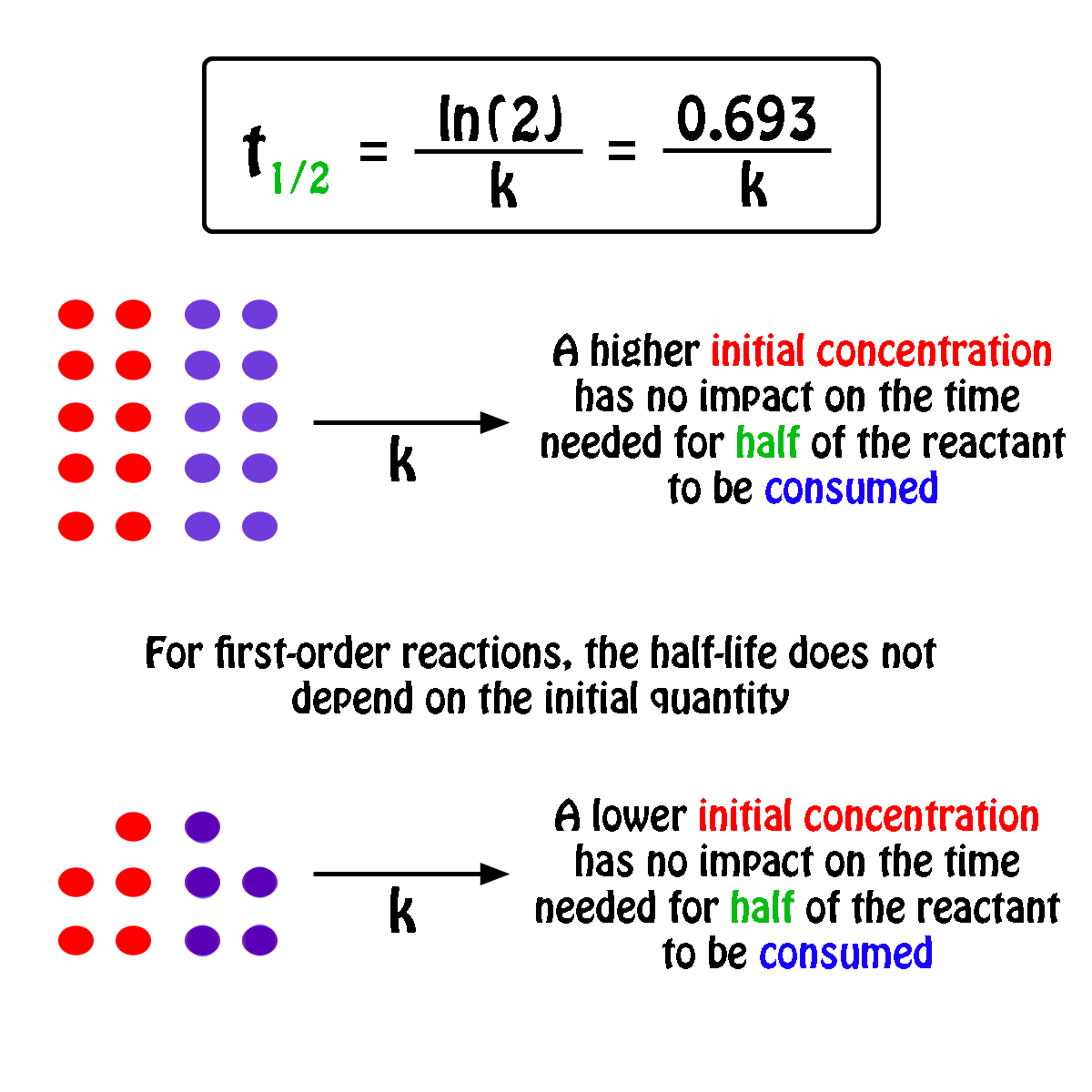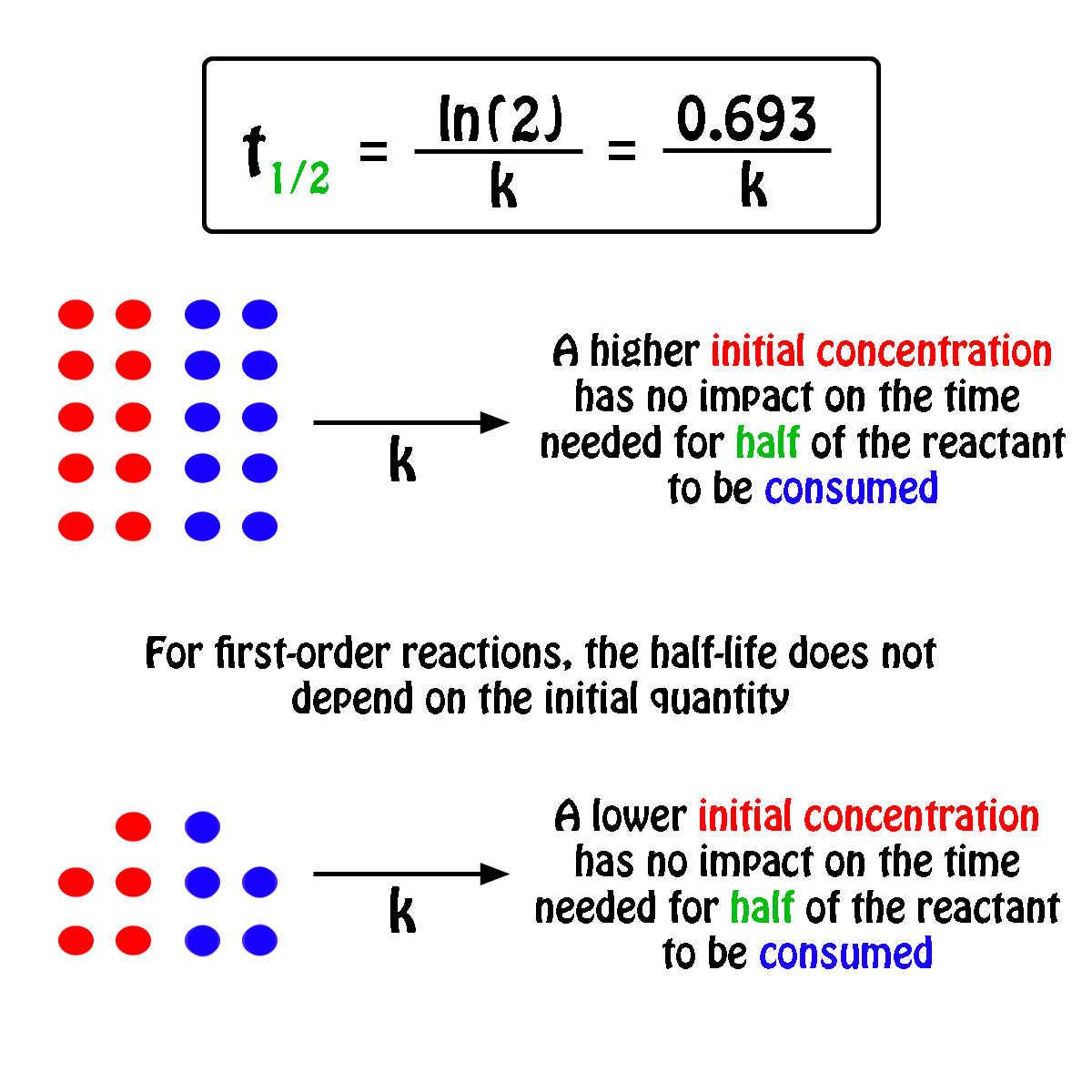
To convert a half life to a rate constant we need to know. Notice that for first order reactions the half life is independent of the initial concentration of reactant which is a unique aspect to first order reactions.
 Integrated Rate Law Problems Zero First Second Order Reactions
Integrated Rate Law Problems Zero First Second Order Reactions
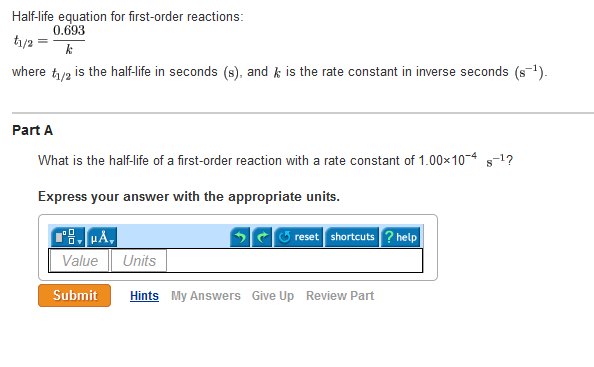
Half life equation for first order reactions where is the half life in seconds and is the rate constant in inverse seconds part awhat is the half life of a first order reaction with a rate constant of 3 10 10 4 part bwhat is the rate constant of a first order reaction that takes 9 20 minutes for the reactant concentration to drop to half of its.

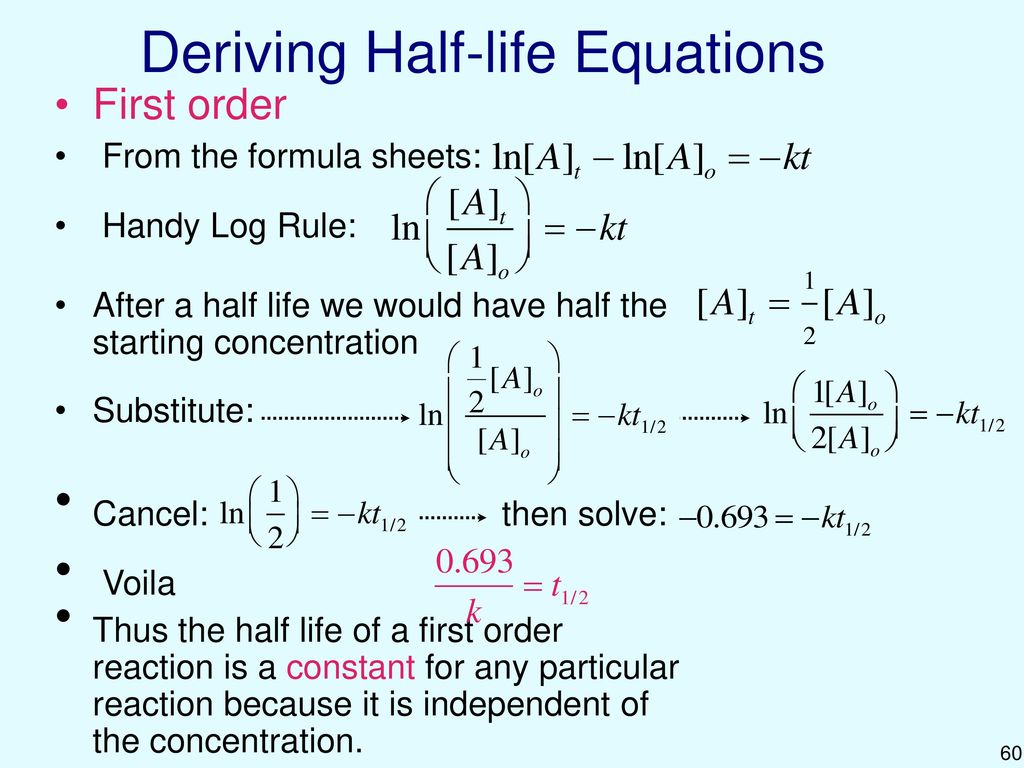
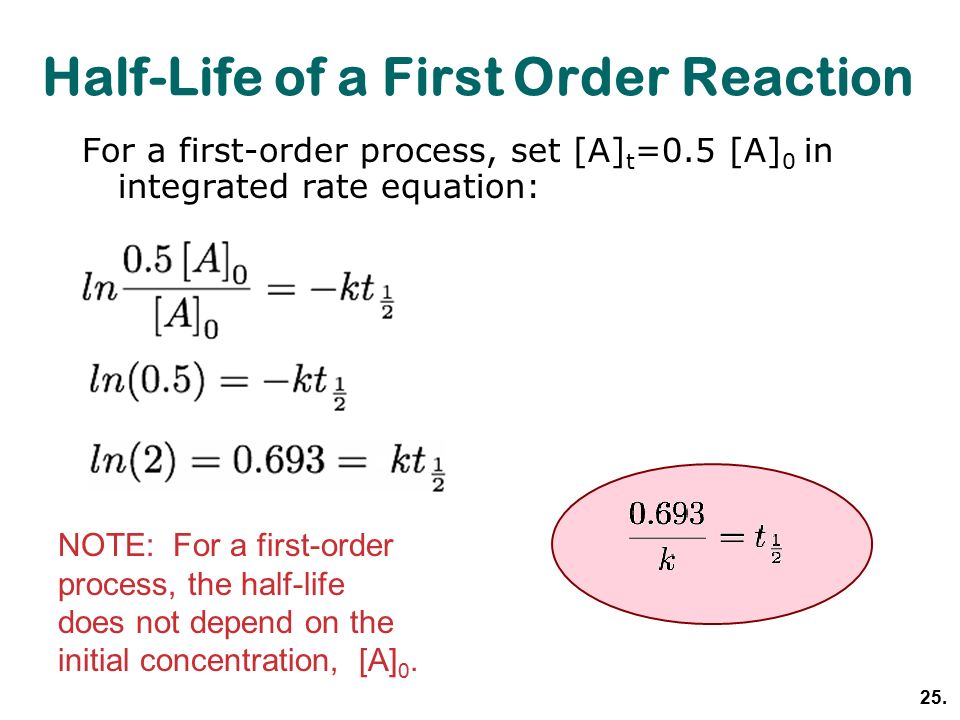
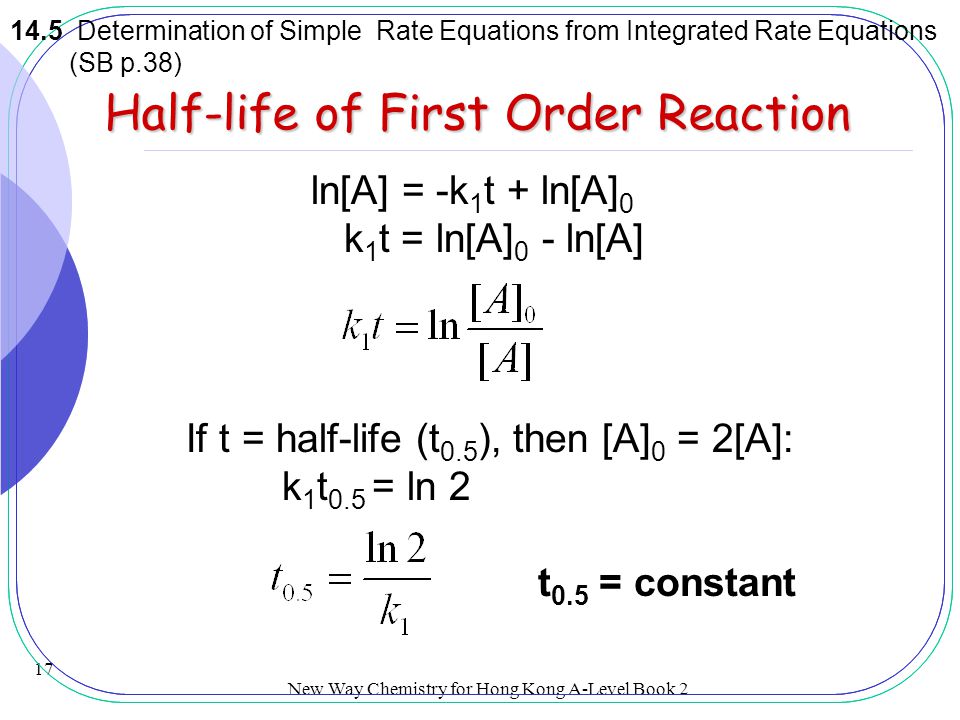
Half life equation first order. For a zero order reaction the mathematical expression that can be employed to determine the half life is. The order of the reaction or enough information to. For a first order reaction the half life is given by.
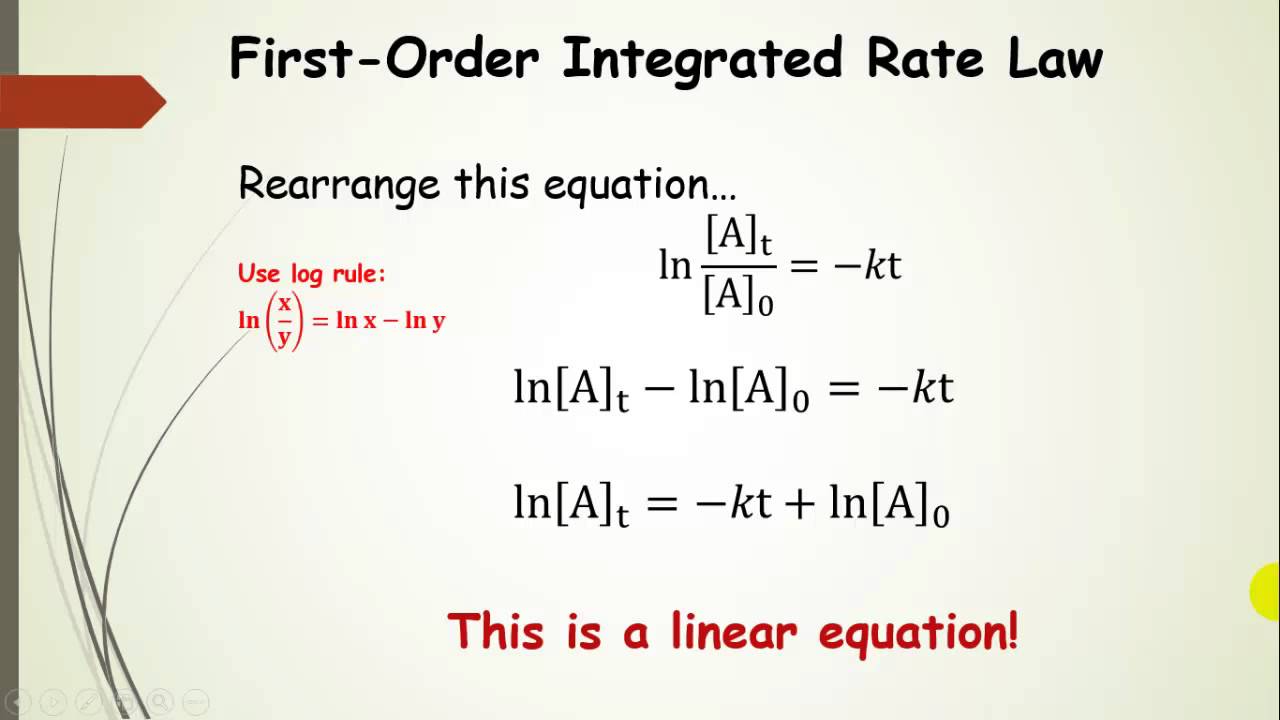
Now let s think about this. And let s think about that for an example. The equations are given above.
Converting a half life to a rate constant. So here is your half life for a first order reaction. The half life of a reaction is the time required for the reactant concentration to decrease to one half its initial value.
Your half life of a first order reaction is independent of the initial concentration of a. Where m stands for concentration in molarity mol l 1 t for time and k for the reaction rate constant. So you re gonna get the same half life.
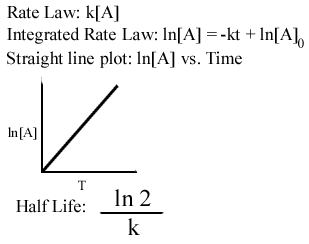
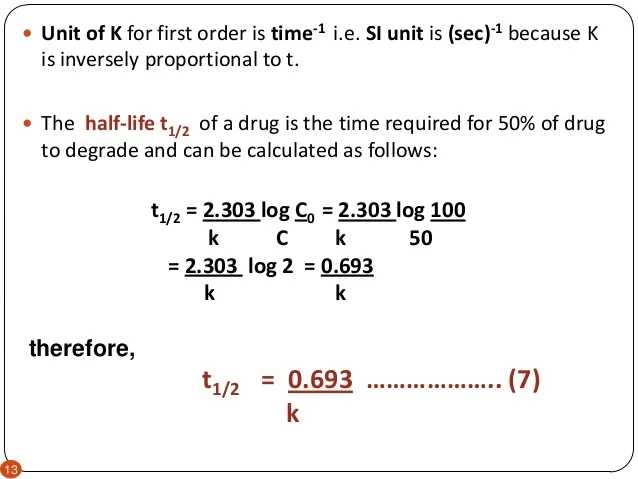
In fractional order reactions the order is a non integer which often indicates a chemical chain reaction or other complex reaction mechanism. For a first order reaction a products. T 1 2 0 693 k.
The half life of a chemical reaction regardless of its order is simply the time needed for half of an initial concentration of a reactant to be consumed by the reaction. And so your half life is constant. If k is a constant obviously 693 is a constant.
T 1 2 r 0 2k. The half life of a first order reaction is a constant that is related to the rate constant for the reaction. The half life of the reaction t.
Substitute this information into the equation for the half life of a reaction with this order and solve for t. Radioactive decay reactions are first order reactions. The practical implication of this is that it takes as much time for a to decrease from 1 m to 0 5 m as it takes for a to decrease from 0 1 m to 0 05 m.
Now a first order reaction is characterized by the fact that the rate of the reaction depends linearly on the concentration of one reactant. The half life of a first order reaction is often expressed as t 1 2 0 693 k as ln 2 0 693. It is important to note that the formula for the half life of a reaction varies with the order of the reaction.
T 1 2 0 693 k.
 Chapter 14 Chemical Kinetics Ppt Video Online Download
Chapter 14 Chemical Kinetics Ppt Video Online Download
Half Life Expressions Chemistnate Lessons
Half Life Expressions Chemistnate Lessons
17 2 Reaction Rates Typically Change With Time Chemistry Libretexts
 Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gct7w6ro9uzjjxwixwfmv Jaxaaouihkr7zd0w Usqp Cau
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gct7w6ro9uzjjxwixwfmv Jaxaaouihkr7zd0w Usqp Cau
 Kinetics Reaction Rates Mechanisms Ppt Download
Kinetics Reaction Rates Mechanisms Ppt Download
 Ppt Integrated Rate Law Powerpoint Presentation Free Download
Ppt Integrated Rate Law Powerpoint Presentation Free Download
Half Life Expressions Chemistnate Lessons
Half Life Of A First Order Reaction Chemistry Assignment
 Chemical Kinetics Chapter Ppt Video Online Download
Chemical Kinetics Chapter Ppt Video Online Download
Half Life Period Of A Reaction Chemical Kinetics
 The Rate Of Reaction Ppt Video Online Download
The Rate Of Reaction Ppt Video Online Download
 Half Life Of A First Order Reaction Video Khan Academy
Half Life Of A First Order Reaction Video Khan Academy
 Rate Equations And Order Of Reactions Ppt Video Online Download
Rate Equations And Order Of Reactions Ppt Video Online Download
 Solved Consider The First Order Reaction Described By The
Solved Consider The First Order Reaction Described By The
 Show That Half Life Of First Order Reaction Is Independent O
Show That Half Life Of First Order Reaction Is Independent O
 The First Order Integrated Rate Law And Half Life Part 4 Youtube
The First Order Integrated Rate Law And Half Life Part 4 Youtube
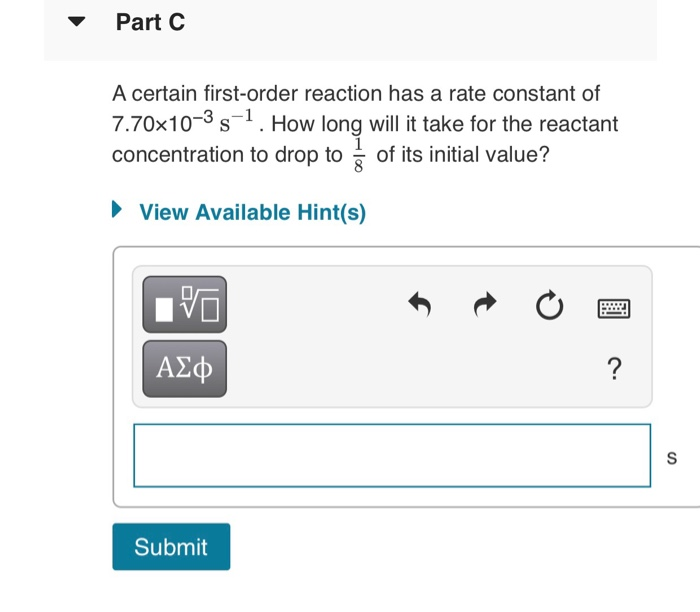
 First Order Reaction Chemistry Problems Half Life Rate Constant
First Order Reaction Chemistry Problems Half Life Rate Constant
 Solved Half Life Equation For First Order Reactions 0 69
Solved Half Life Equation For First Order Reactions 0 69
 First Order Kinetic Equation Google Search Words Word Search
First Order Kinetic Equation Google Search Words Word Search
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctgyzqelmrmig7umh Tfyxgkozh5aeueohhu5xis49fcp22olpg Usqp Cau
 Answered The Half Life Of A First Order Reaction Bartleby
Answered The Half Life Of A First Order Reaction Bartleby
 Ppt Summary Of The Kinetics Of Zero Order First Order And
Ppt Summary Of The Kinetics Of Zero Order First Order And
 Learn Chemistry Tutorials Kinetics Tutorial
Learn Chemistry Tutorials Kinetics Tutorial
 Applications Of First Order Differential Equations Exponential
Applications Of First Order Differential Equations Exponential
 Solved Half Life Equation For First Order Reactions T1 2
Solved Half Life Equation For First Order Reactions T1 2
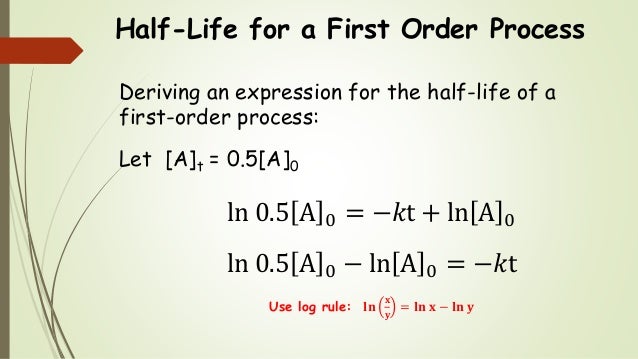 Chem 2 Chemical Kinetics Iv The First Order Integrated Rate Law
Chem 2 Chemical Kinetics Iv The First Order Integrated Rate Law
 Solved Half Life Equation For First Order Reactions T 2
Solved Half Life Equation For First Order Reactions T 2
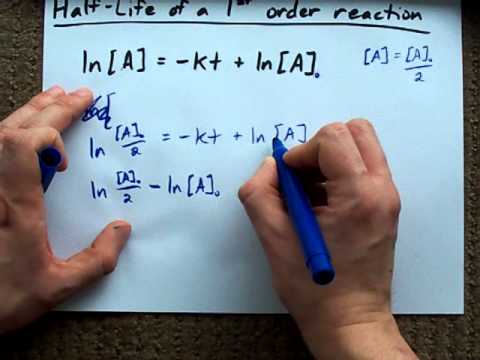 Half Life Of A First Order Reaction Derivation Youtube
Half Life Of A First Order Reaction Derivation Youtube
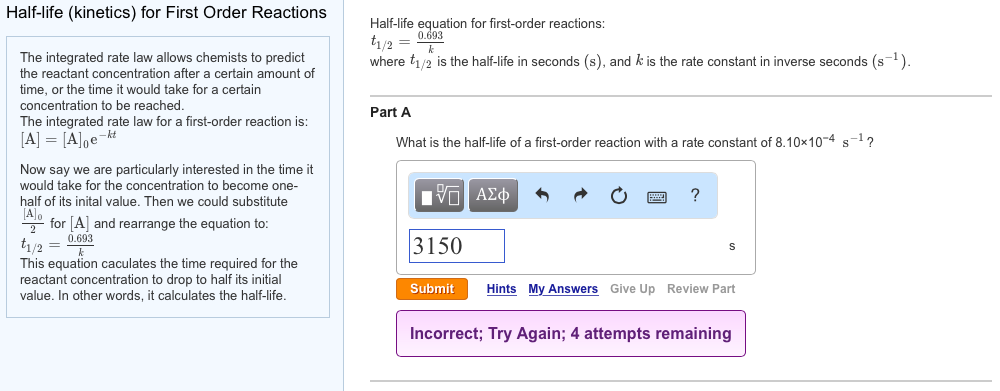 Solved Half Life Kinetics For First Order Reactions Hal
Solved Half Life Kinetics For First Order Reactions Hal
 Regression Equation And Half Life For First Order Dissipation Of
Regression Equation And Half Life For First Order Dissipation Of
 Solved Consider The First Order Reaction Described By The
Solved Consider The First Order Reaction Described By The
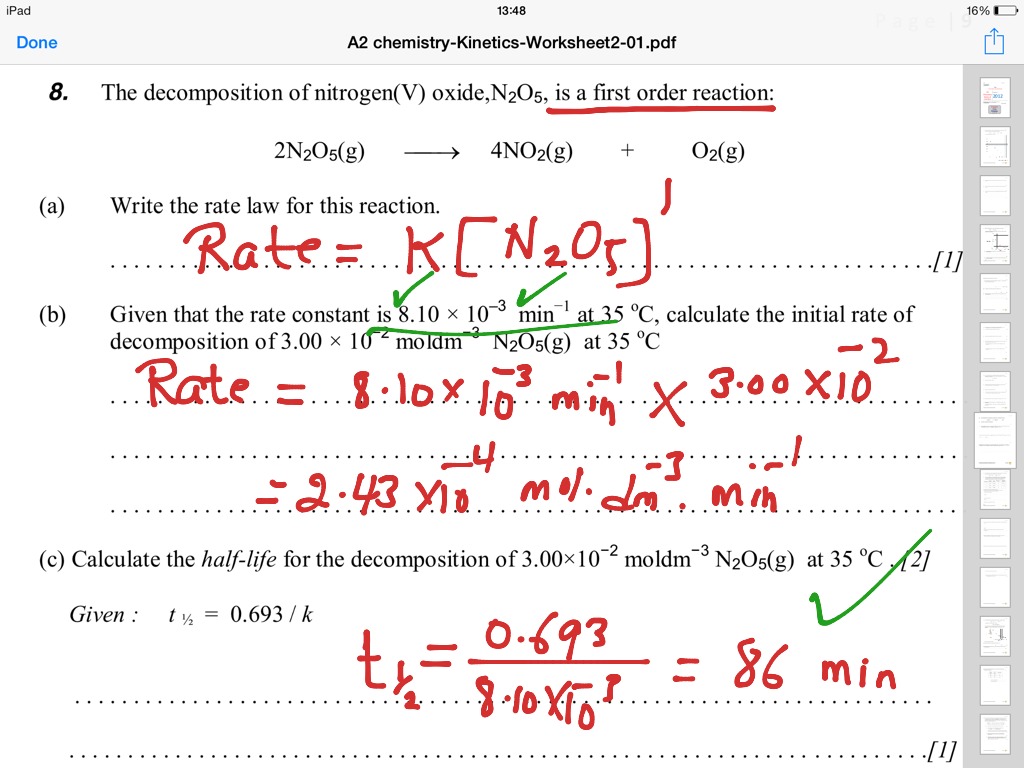 Finding Half Life For A First Order Reaction Science Chemistry
Finding Half Life For A First Order Reaction Science Chemistry
 Half Life Of First Order Reaction Youtube
Half Life Of First Order Reaction Youtube

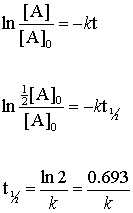
Posting Komentar
Posting Komentar