What Is Polar Covalent Bond In Chemistry
A polar covalent bond is a bond that is formed due to the unequal sharing of electrons between two partially charged atoms. Since the molecule is polar water is a polar solvent also.
Any covalent bond between atoms of different elements is a polar bond but the degree of polarity varies widely.
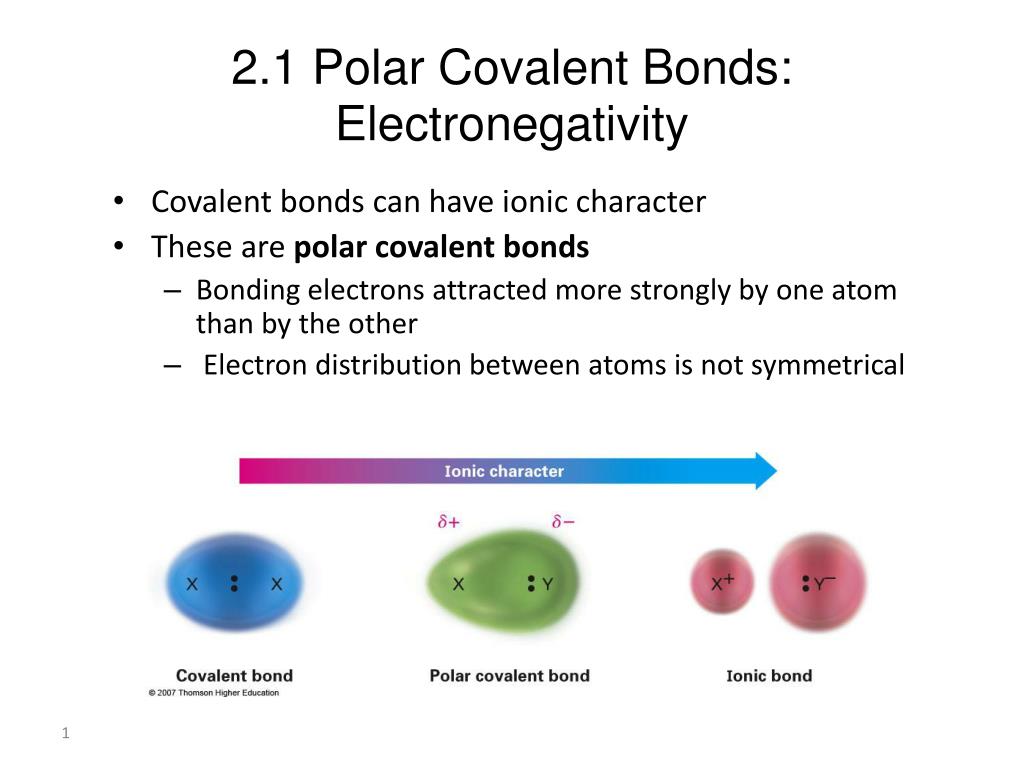
What is polar covalent bond in chemistry. Consider the hydrogen chloride hcl molecule. In this lesson you will learn about two. A dipoleforms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge.
This causes the molecule to have a slight electrical dipole moment where one end is slightly positive and the other is slightly negative. Polar molecules polar molecules occur when two atoms do not share electrons equally in a covalent bond. Some bonds between different elements are only minimally polar while others are strongly polar.
A polar covalent bond is a type of bond between two or more atoms in which the atoms do not share their pair of electrons equally. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. Chlorine has a higher electronegativity than hydrogen but the chlorine atom s attraction for electrons is not sufficient to remove an electron.
We can also say that it is the dividing line between the formation of a pure covalent bond and an ionic bond. Each atom in hcl requires one more electron to form an inert gas electron configuration. However if we want to define it more accurately a polar covalent bond is a bond that exists between two atoms consisting of electrons that are unevenly distributed.
In a polar covalent bond sometimes simply called a polar bond the distribution of electrons around the molecule is no longer symmetrical. Polar covalent bond explained. This is observed when the difference in electronegativity between the bonding atoms is between 0 5 and 1 7.
Did you know that some types of chemical bonds behave similarly to the way that children play with toys. This happens when there is a difference between the electronegativityof each atom. In general a polar bond is a certain class of a covalent bond.
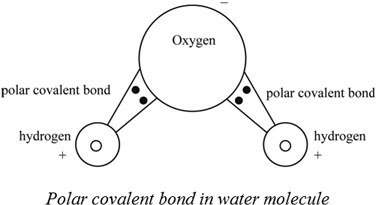
In this type of bond one of the atoms is stronger than the other and attracts the electrons so that they spend more time closer to the stronger atom. Water is a polar molecule because the electrons are unevenly distributed. Ionic bonds can be considered the ultimate in polarity with electrons being transferred rather than shared.
A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed.
How Does A Polar Covalent Bond Differ From An Nonpolar Socratic
 Polarity Dipoles And Bonds Principles Of Structural Chemistry
Polarity Dipoles And Bonds Principles Of Structural Chemistry
 Definition Of Polar Covalent Bond Chegg Com
Definition Of Polar Covalent Bond Chegg Com
Illustrated Glossary Of Organic Chemistry Polar Covalent Bond
 Chemical Bonds Anatomy And Physiology I
Chemical Bonds Anatomy And Physiology I
 How Are Polar Covalent Bonds Formed Quora
How Are Polar Covalent Bonds Formed Quora
 Polar And Nonpolar Covalent Bonds And Molecules Ppt Video Online
Polar And Nonpolar Covalent Bonds And Molecules Ppt Video Online
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctmolizrals07yyegr Irwadp0vj Wmernolsaf5flwcmojckfo Usqp Cau
 Polar Vs Nonpolar Bonds Overview Examples Expii
Polar Vs Nonpolar Bonds Overview Examples Expii
Covalent And Ionic Bonds Course Hero
 Polar Covalent Bond Polar Covalent Bond Examples Covalent
Polar Covalent Bond Polar Covalent Bond Examples Covalent
 Polar And Nonpolar Covalent Bonds Definitions And Examples
Polar And Nonpolar Covalent Bonds Definitions And Examples
 Polar Covalent Bond Chemistry Stack Exchange
Polar Covalent Bond Chemistry Stack Exchange
 4 2 Polar And Non Polar Covalent Bonds Sl Youtube
4 2 Polar And Non Polar Covalent Bonds Sl Youtube
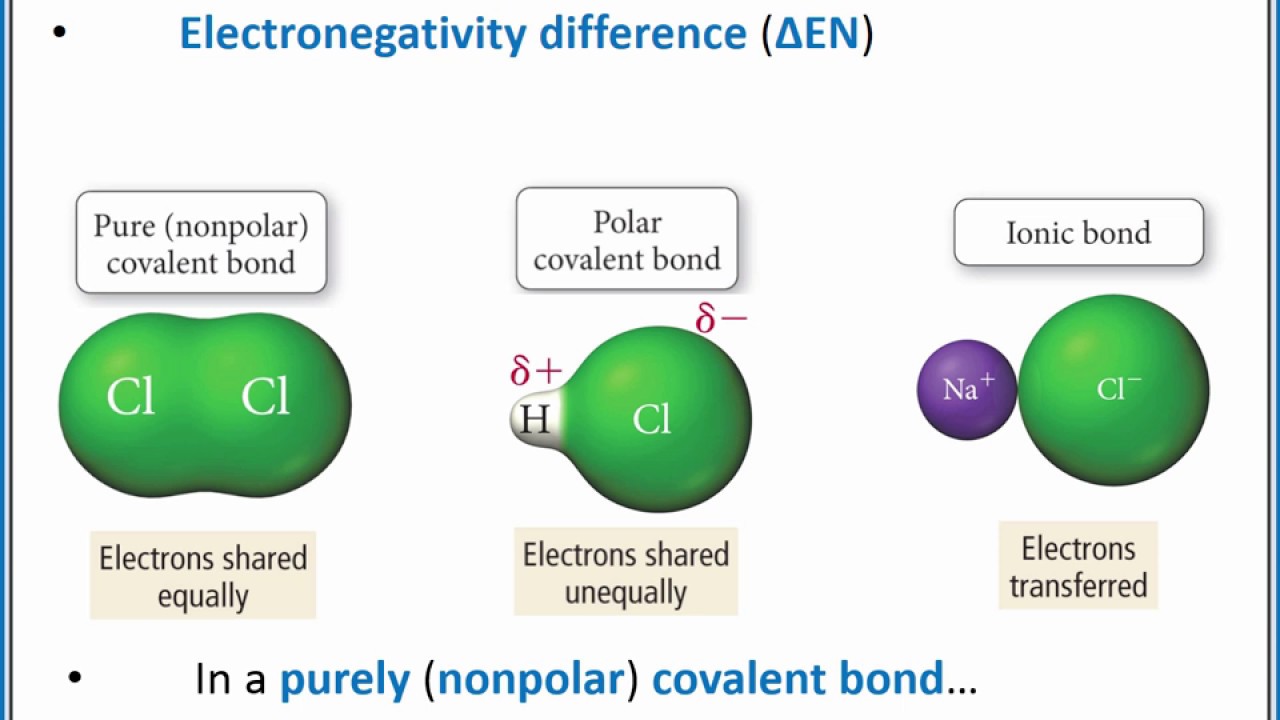 Chemistry 101 Using Electronegativity To Classify Bonds As Polar
Chemistry 101 Using Electronegativity To Classify Bonds As Polar
Chemical Bonds Iii Polar Covalent Video Lesson Transcript
 Polar Vs Nonpolar Bonds Overview Examples Expii
Polar Vs Nonpolar Bonds Overview Examples Expii
 Covalent Bonds Biology For Majors I
Covalent Bonds Biology For Majors I
 Unit 6 Properties Of Covalent Compounds Flashcards Quizlet
Unit 6 Properties Of Covalent Compounds Flashcards Quizlet
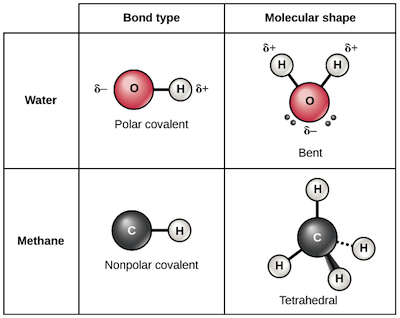 Chemical Bonds Chemistry Of Life Biology Article Khan Academy
Chemical Bonds Chemistry Of Life Biology Article Khan Academy
 Chemistry Not Mystery Polar Covalent Bond Water
Chemistry Not Mystery Polar Covalent Bond Water
 Chemical Bonds Principles Of Biology
Chemical Bonds Principles Of Biology

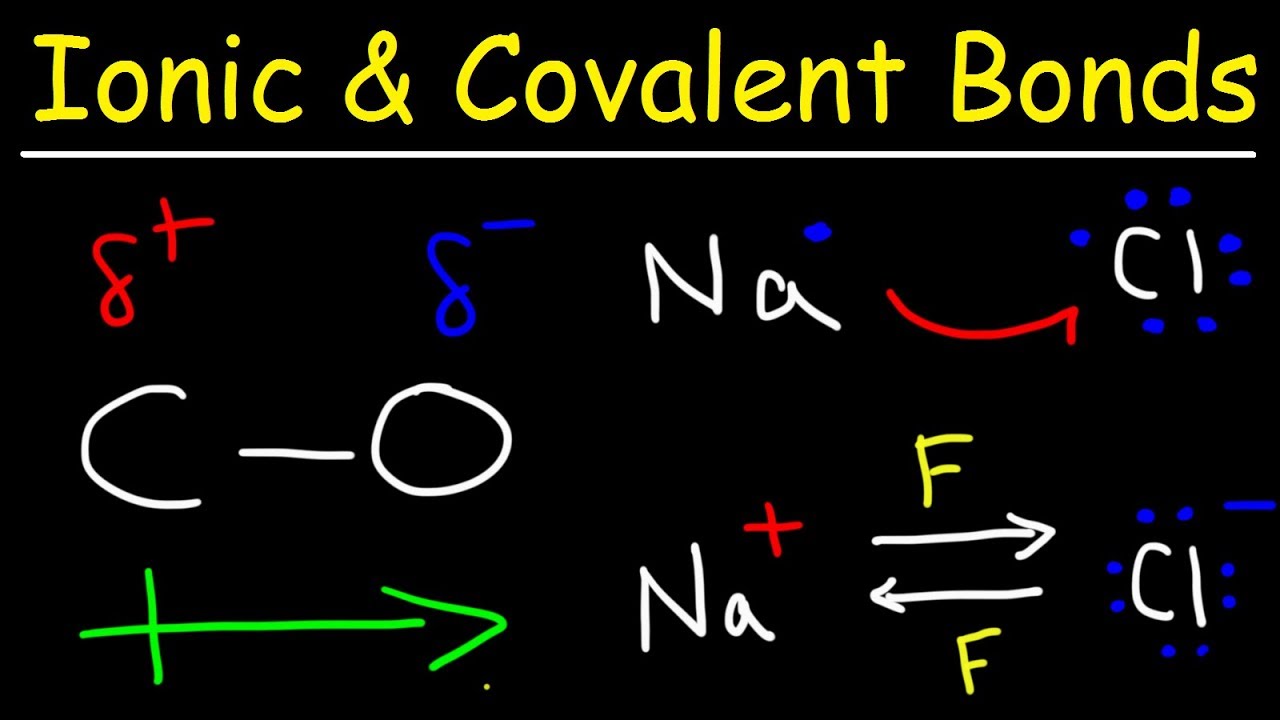 Ionic Bonds Polar Covalent Bonds And Nonpolar Covalent Bonds
Ionic Bonds Polar Covalent Bonds And Nonpolar Covalent Bonds
Difference Between Non Polar And Polar Covalent Bonds Difference
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg) Definition And Examples Of A Polar Bond In Chemistry
Definition And Examples Of A Polar Bond In Chemistry
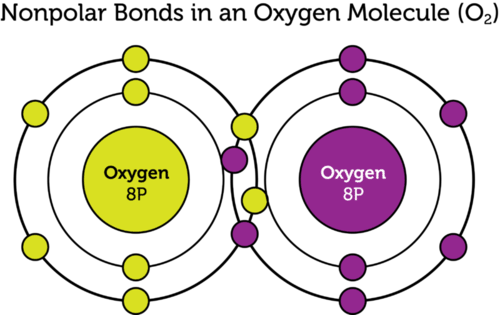
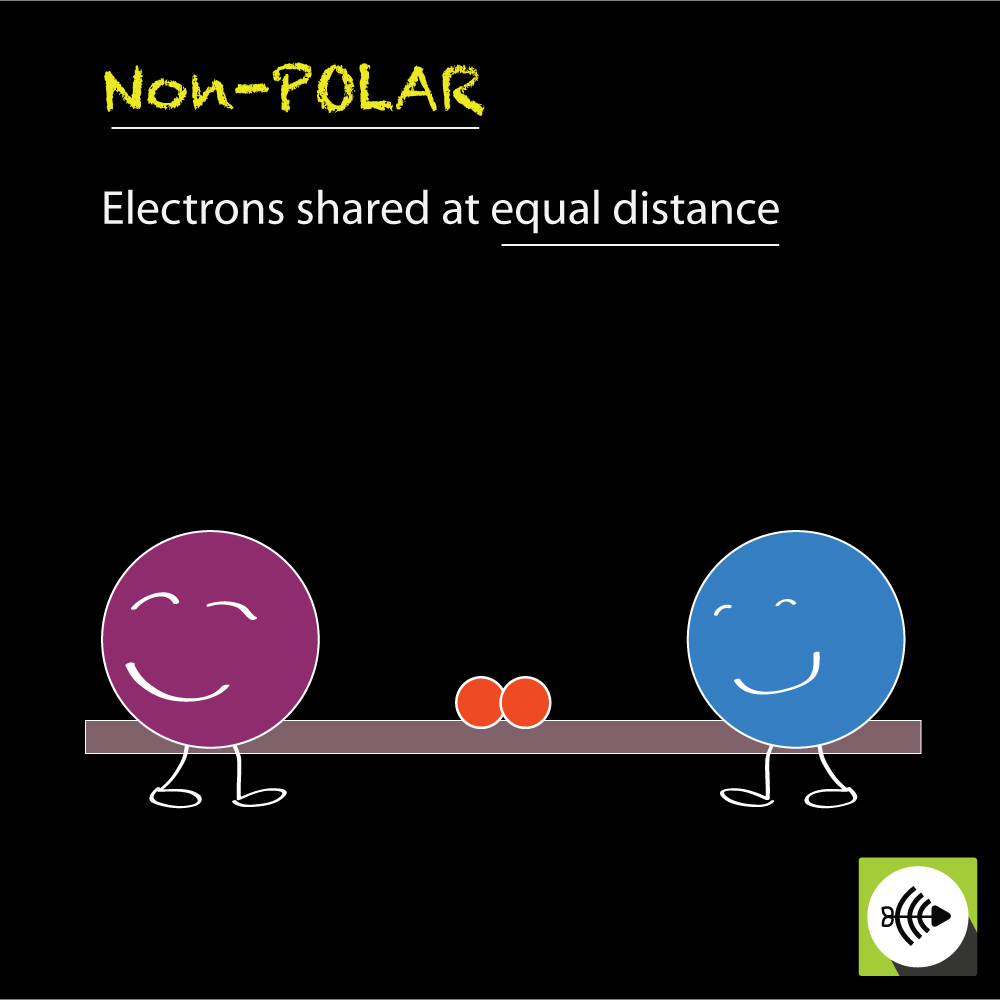 What Is Nonpolar Covalent Bond
What Is Nonpolar Covalent Bond
 E Book 02 Chemical Bonds Polar Covalent Bonds
E Book 02 Chemical Bonds Polar Covalent Bonds
 Ppt 2 1 Polar Covalent Bonds Electronegativity Powerpoint
Ppt 2 1 Polar Covalent Bonds Electronegativity Powerpoint
 Polar And Nonpolar Covalent Bonds Definitions And Examples
Polar And Nonpolar Covalent Bonds Definitions And Examples
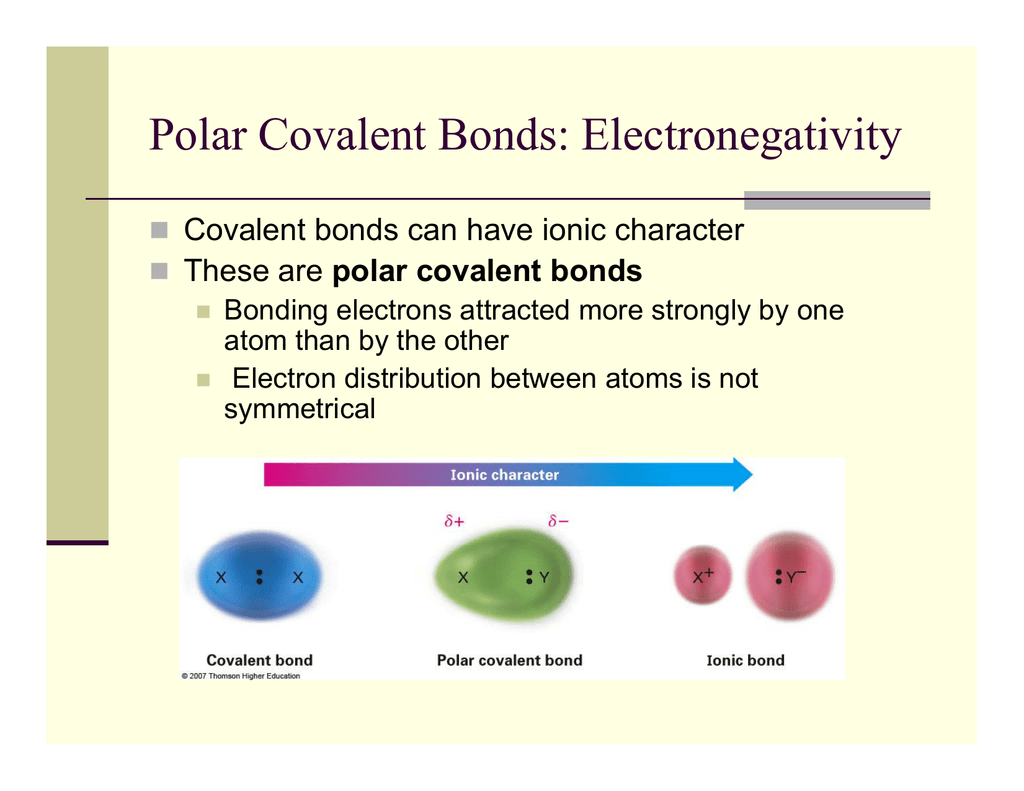 Polar Covalent Bonds Electronegativity
Polar Covalent Bonds Electronegativity
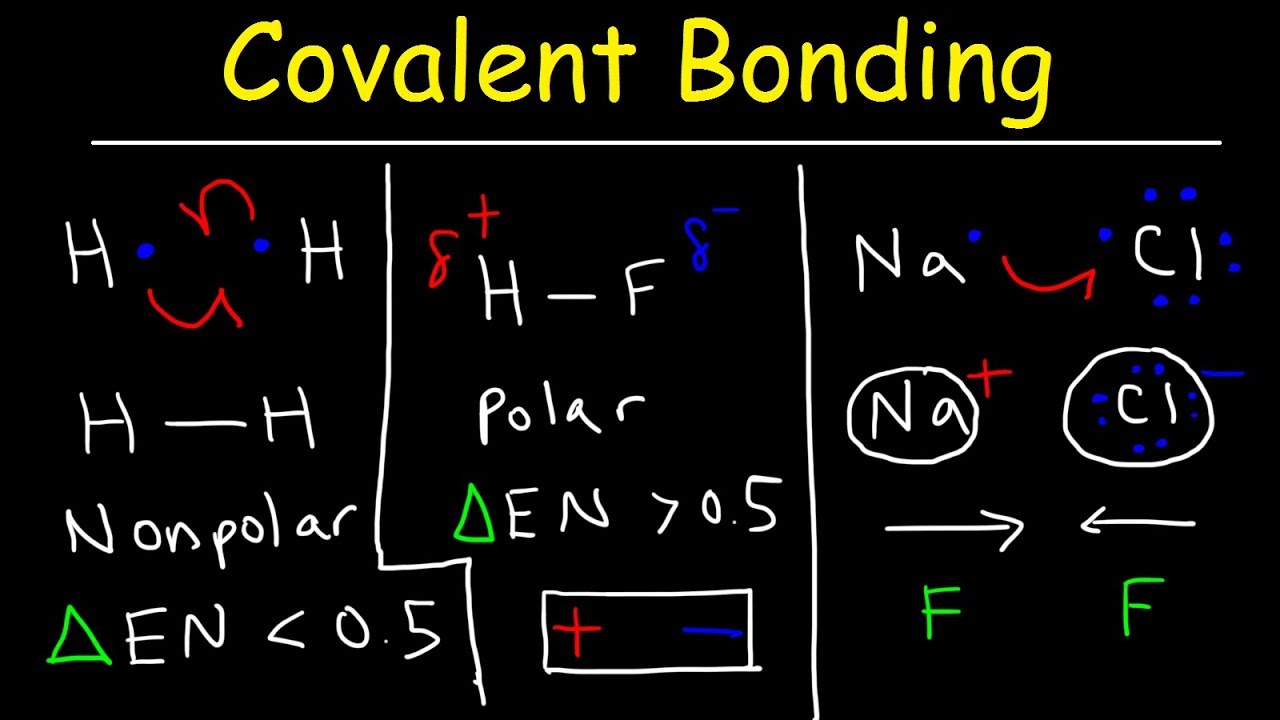 Polar Covalent Bonds And Nonpolar Covalent Bonds Ionic Bonding
Polar Covalent Bonds And Nonpolar Covalent Bonds Ionic Bonding
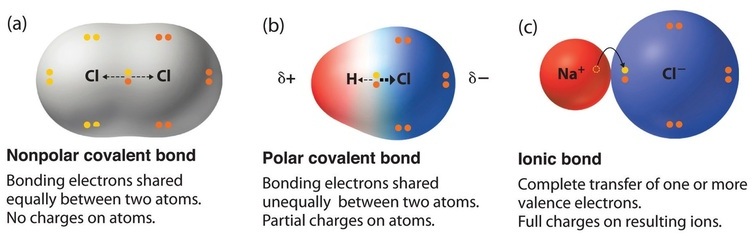 9 3 Molecular Shape And Molecular Polarity Chemistry Libretexts
9 3 Molecular Shape And Molecular Polarity Chemistry Libretexts
 Polar And Nonpolar Covalent Bonds Definitions And Examples
Polar And Nonpolar Covalent Bonds Definitions And Examples
Compare Ionic And Covalent Compounds

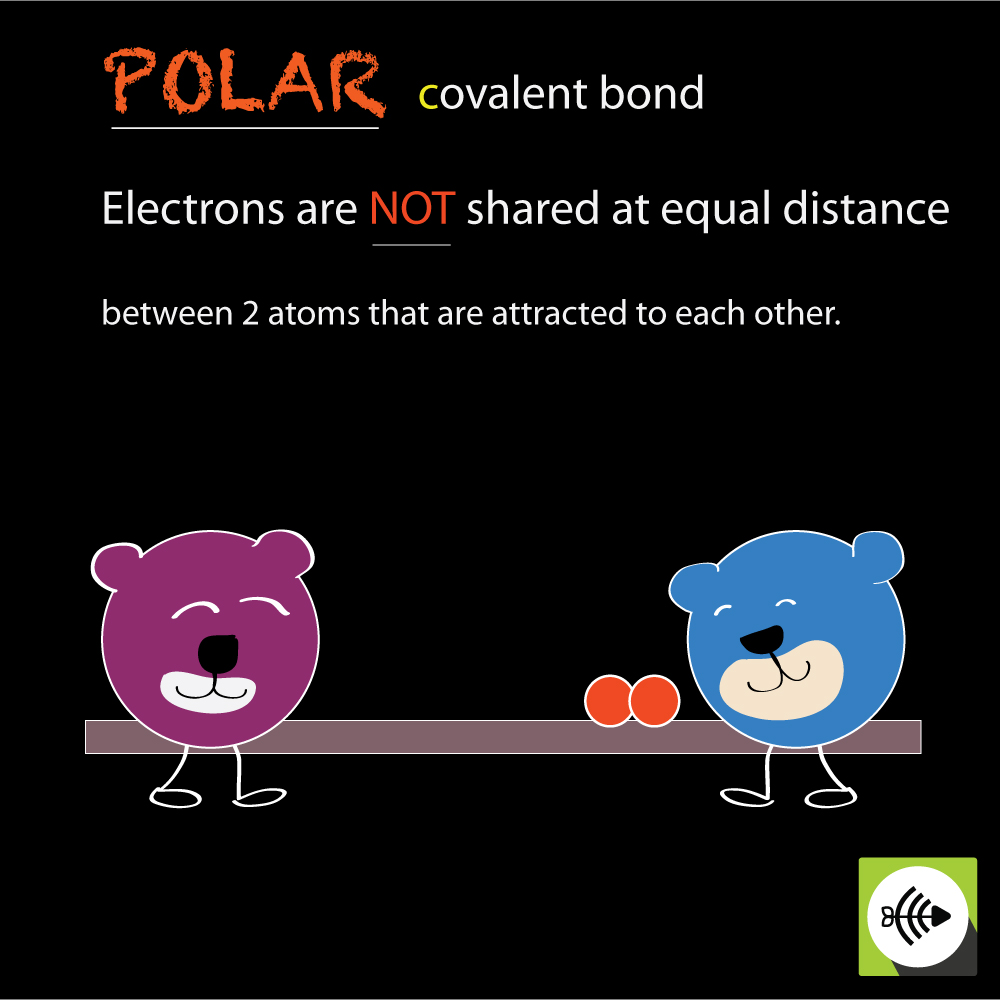
Posting Komentar
Posting Komentar