Molecular Vs Empirical Formula
Empirical formulas are the simplest form of formulas that we can write for a molecule while molecular formulas are the formulas showing the type of atoms and number of each atom connected in the molecule. Molecular formula last example shows difference between empirical and molecular formulas.
 Empirical And Molecular Formulas Of Compounds Mole And Empirical
Empirical And Molecular Formulas Of Compounds Mole And Empirical
Thus the actual chemical formula is.
Molecular vs empirical formula. If the formula is simplified then it is an empirical formula. The empirical formula of a compound gives the simplest ratio of the number of different atoms present whereas the molecular formula gives the actual number of each different atom present in a molecule. If we multiplied our empirical formula by 2 then the molecular mass would be correct.
Hence the key difference between empirical and molecular formulas is that empirical formula only gives the simplest ratio of atom whereas molecular formula gives the exact number of each atom in a molecule. 2 c 3 h 4 o 3 c6h8o6. Created by sal khan.
Molecular formula of a compound gives the actual number of atoms of each element present in one molecule of the. If a compound s molecular. Simplest possible formula with correct ratios of atoms molecular formula.
The molecular formula needs to be a multiple of the empirical formula. The empirical formula is not used for naming purposes as an empirical formula can result in any number of molecular formulas. The empirical formula contains the most simplified ratio of the moles of elements in the compound.
The empirical formula is not often used in reaction schemes. Empirical formula of a compound gives the simplest whole number ratio of atoms of each element present in the compound. Formula showing the actual composition of a molecule can find molecular formula from empirical formula if we know molar mass.
It is standard for many ionic compounds like calcium chloride cacl 2 and for macromolecules such as silicon dioxide sio 2. A empirical formula b molecular formula. The formulas for water and hydrogen peroxide are.
If you can divide all of the numbers in a molecular formula by some value to simplify them further then the empirical or simple formula will be different from the molecular formula. An empirical formula makes no mention of the arrangement or number of atoms. Empirical formulas show the simplest whole number ratio of atoms in a compound molecular formulas show the number of each type of atom in a molecule and structural formulas show how the atoms in a molecule are bonded to each other.
Thus it would appear that our empirical formula is essentially one half the mass of the actual molecular mass. Molecular formulas tell you how many atoms of each element are in a compound and empirical formulas tell you the simplest or most reduced ratio of elements in a compound. The molecular formula on the other hand shows the number of each type of atom in a molecule.
There are three main types of chemical formulas. The molecular formula is commonly used in reactions and other chemical recordings. Glucose has 2 moles of hydrogen for every mole of carbon and oxygen.
The molecular formula is commonly used and is a multiple of the empirical formula. Empirical molecular and structural. A compound can be represented by two types of chemical formulae.
The empirical formula for glucose is ch 2 o.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsyxqgzrpsxomf6woq Dnja Pa0z3vzmqs Pqiqil7owmk7 Y O Usqp Cau
Percent Composition And Empirical And Molecular Formulas Chemistry
 1 1 The Mole Concept 1 2 Formulas Assessment Objectives
1 1 The Mole Concept 1 2 Formulas Assessment Objectives
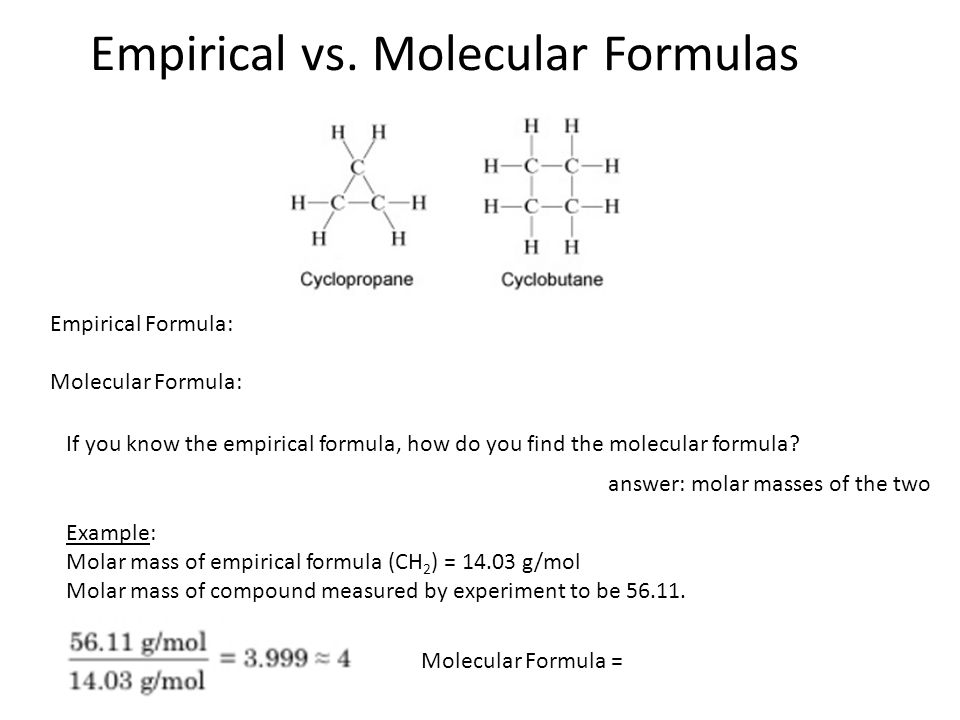 Section 3 2 Stoichiometry And Compound Formulas Ppt Video Online
Section 3 2 Stoichiometry And Compound Formulas Ppt Video Online
Mdhssch3u Empirical And Molecular Formula
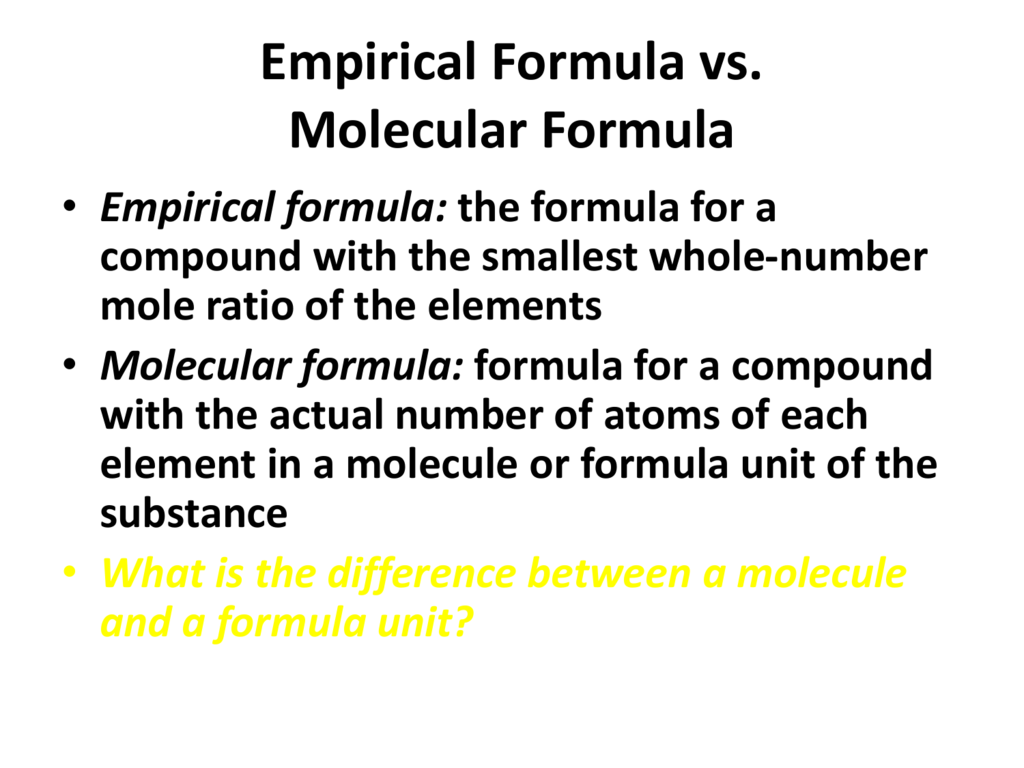 Empirical Formula Vs Molecular Formula
Empirical Formula Vs Molecular Formula
Pre Ap Chemistry Empirical Formulas Vs Molecular Formulas
 Classic Chemistry Finding The Empirical Formula Www
Classic Chemistry Finding The Empirical Formula Www
 Determining Empirical And Molecular Formulas Chemistry Tutorial
Determining Empirical And Molecular Formulas Chemistry Tutorial
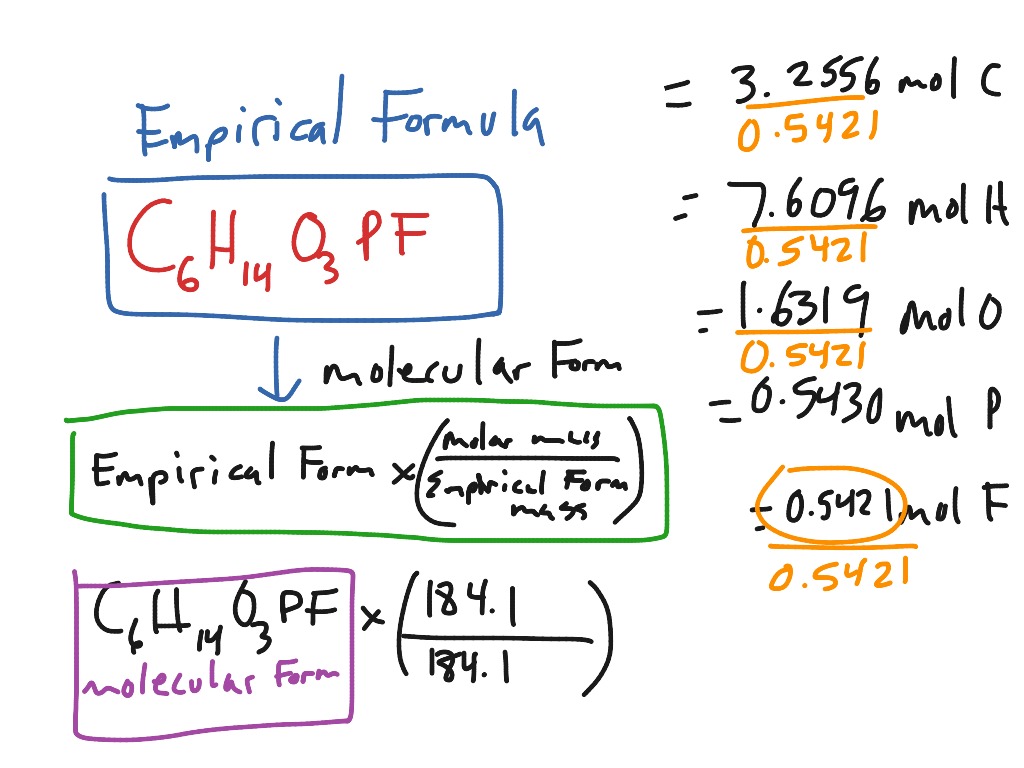 Empirical And Molecular Formulas 6 Science Chemistry Empirical
Empirical And Molecular Formulas 6 Science Chemistry Empirical
 Ppt Empirical And Molecular Formula Notes Powerpoint
Ppt Empirical And Molecular Formula Notes Powerpoint
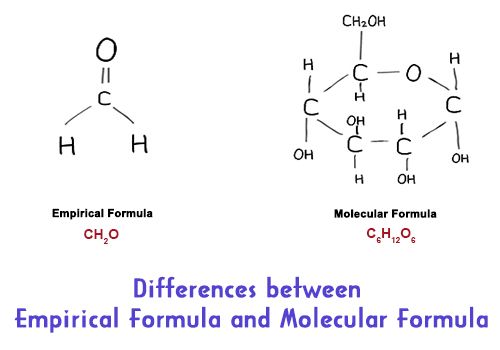 Differences Between Empirical Formula And Molecular Formula Qs Study
Differences Between Empirical Formula And Molecular Formula Qs Study
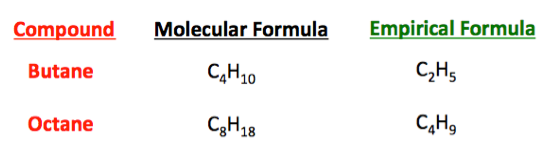 What Is The Empirical Formula Of H2o2 Socratic
What Is The Empirical Formula Of H2o2 Socratic
Section 6 5 Emperical Versus Molecular Formulas
:max_bytes(150000):strip_icc()/Molecular-formula-58e51cdc3df78c5162a9e340.jpg) Calculate Empirical And Molecular Formulas
Calculate Empirical And Molecular Formulas
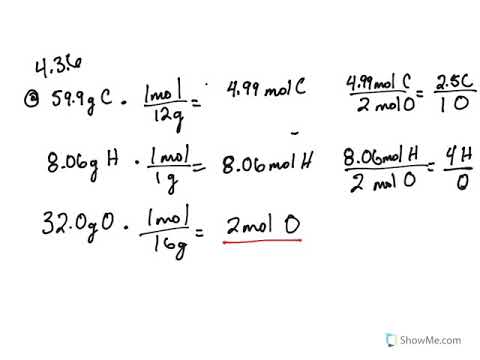 4 3 Empirical And Molecular Formulas Problems Chemistry
4 3 Empirical And Molecular Formulas Problems Chemistry
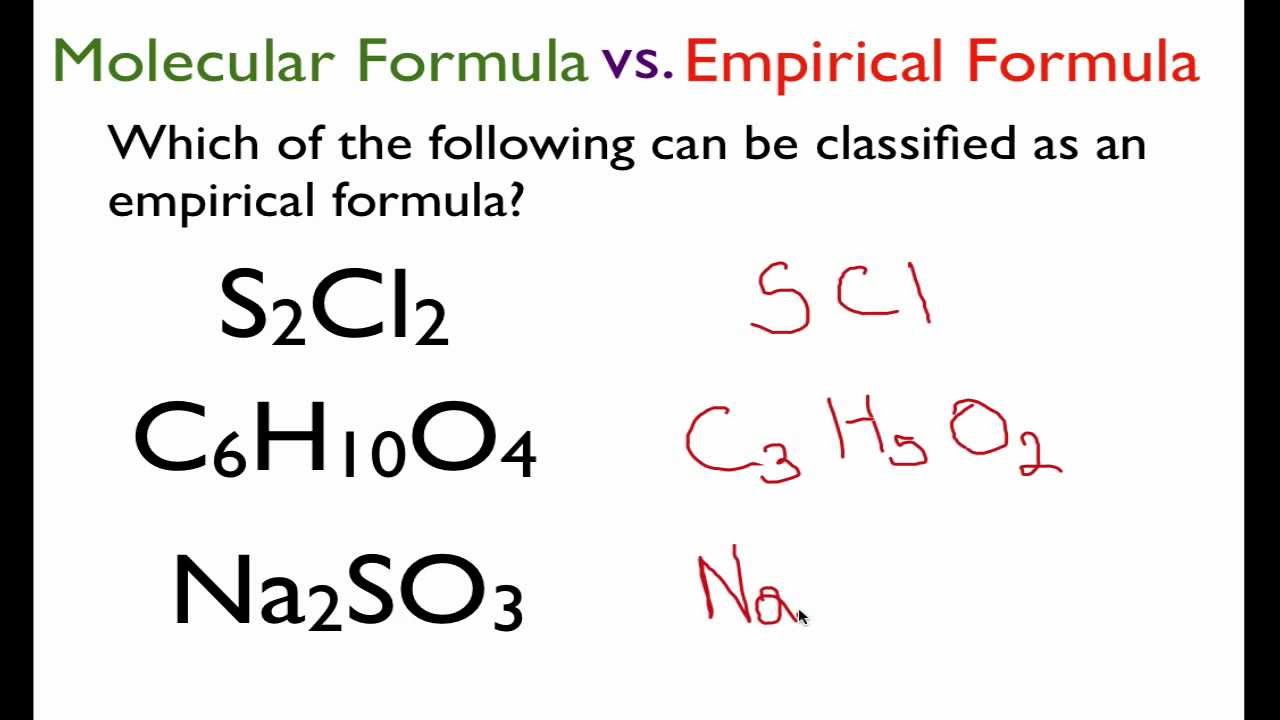 From The Molecular Formula To The Empirical Formula Youtube
From The Molecular Formula To The Empirical Formula Youtube
 What Is The Empirical Formula Of Melamine Socratic
What Is The Empirical Formula Of Melamine Socratic
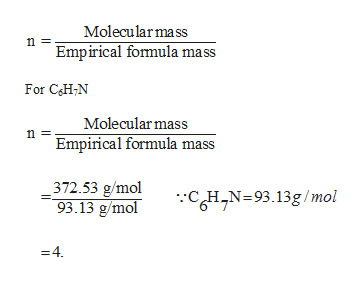 Answered From The Given Empirical Formula And Bartleby
Answered From The Given Empirical Formula And Bartleby
Determining Empirical And Molecular Formulas Lessons Tes Teach
Empirical Formula And Molecular Formula Calculations For Mcat
 Calculating Empirical Formulas Ppt Video Online Download
Calculating Empirical Formulas Ppt Video Online Download
Section 6 5 Emperical Versus Molecular Formulas
 Empirical Vs Molecular Formula
Empirical Vs Molecular Formula
 Empirical Molecular And Structural Formulas Video Khan Academy
Empirical Molecular And Structural Formulas Video Khan Academy
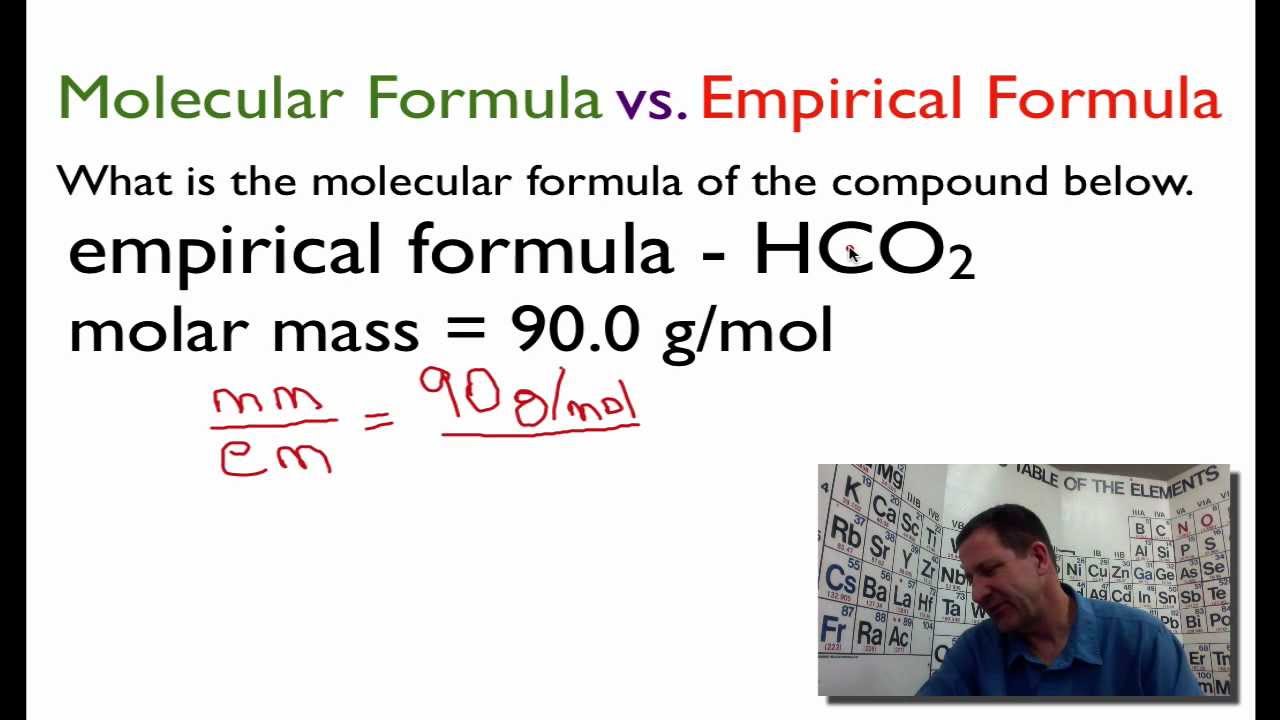 From The Empirical Formula To The Molecular Formula Youtube
From The Empirical Formula To The Molecular Formula Youtube
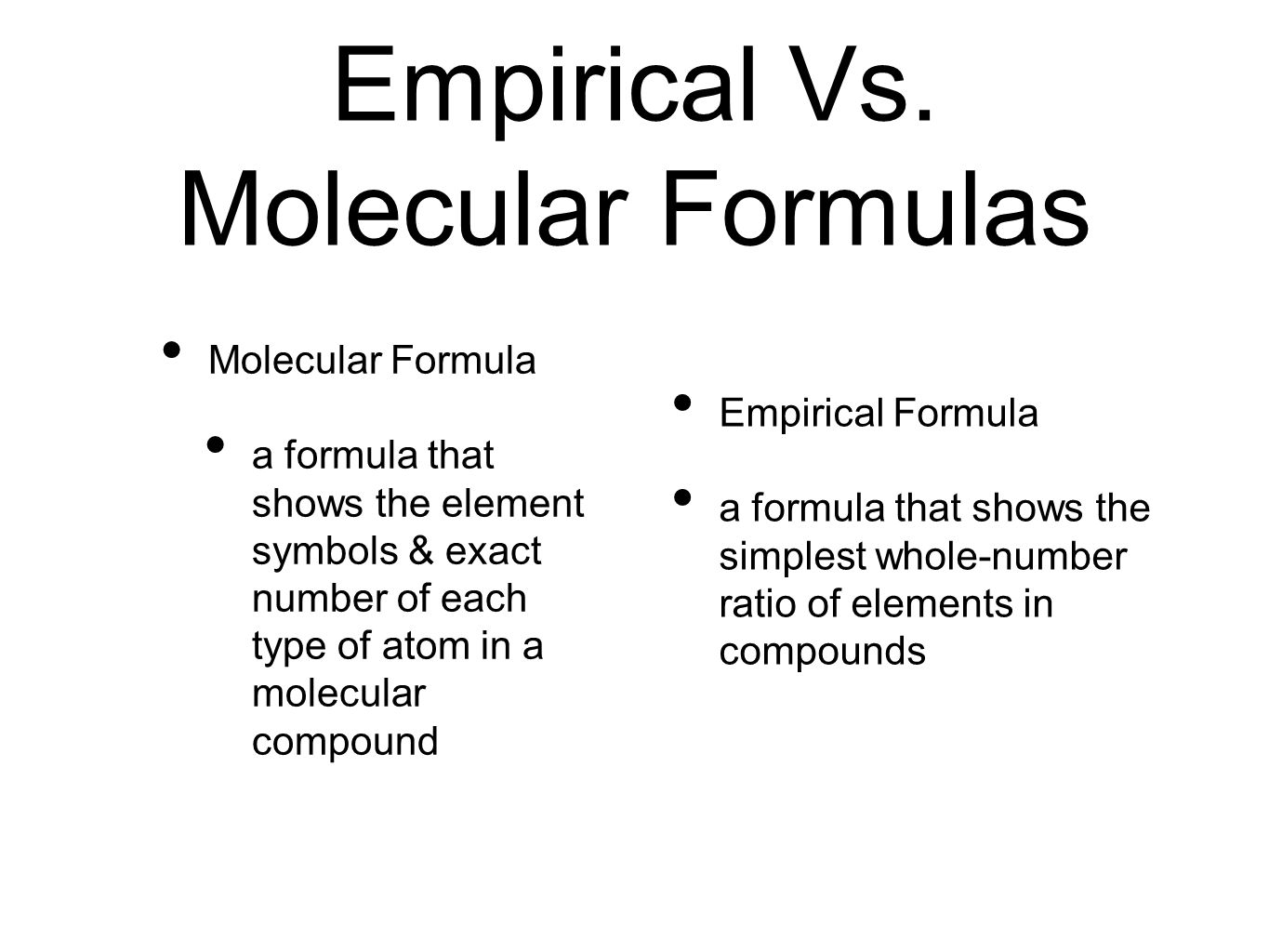 Empirical Formula Molecular Formula Ppt Download
Empirical Formula Molecular Formula Ppt Download
 Structure Of Pga Its Molecular Formula Empirical Formula And
Structure Of Pga Its Molecular Formula Empirical Formula And
 Difference Between Empirical And Molecular Formula Teaching
Difference Between Empirical And Molecular Formula Teaching
What Is An Empirical Formula Quora
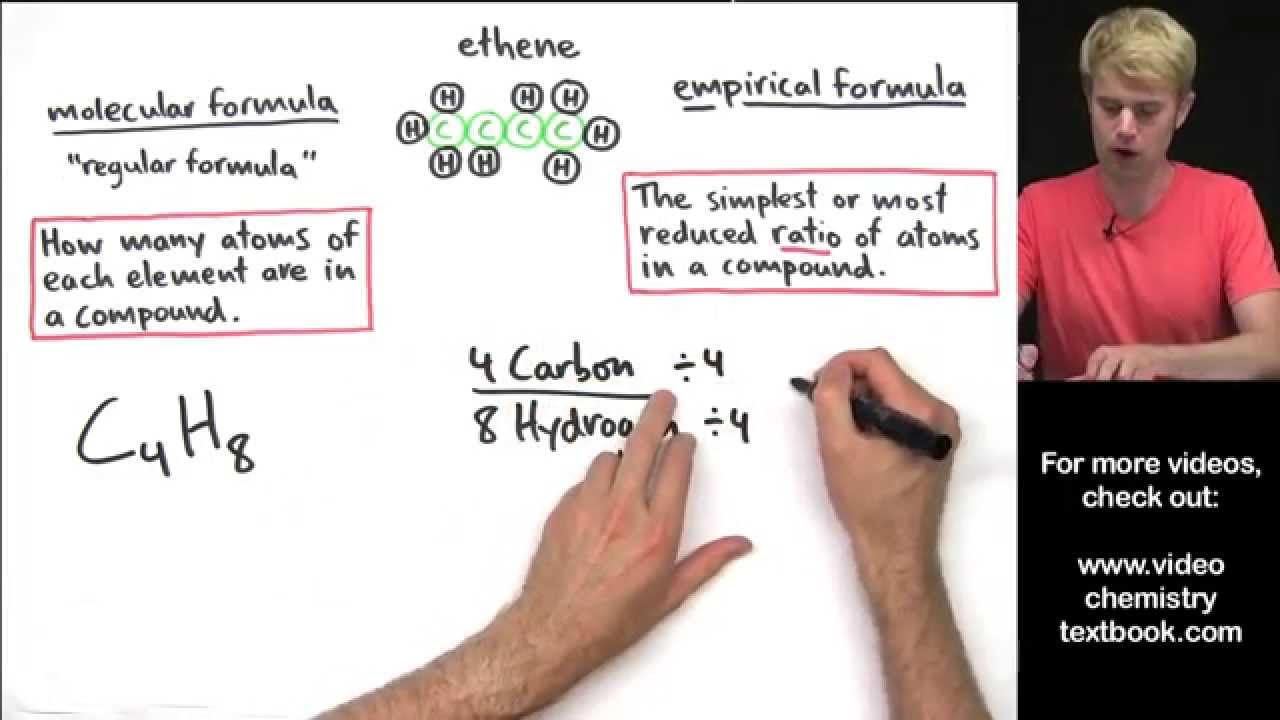 Empirical Formula And Molecular Formula Introduction Youtube
Empirical Formula And Molecular Formula Introduction Youtube
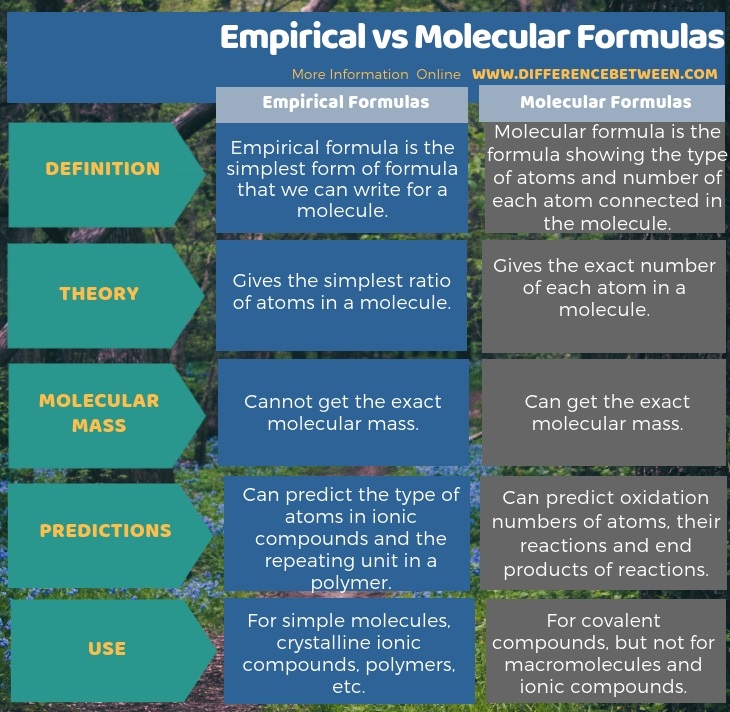 Difference Between Empirical And Molecular Formulas Compare The
Difference Between Empirical And Molecular Formulas Compare The
 Empirical And Molecular Formula Notes
Empirical And Molecular Formula Notes
 Empirical Vs Molecular Formulas Worksheet For 9th Higher Ed
Empirical Vs Molecular Formulas Worksheet For 9th Higher Ed

 Unit V The Mole Concept V 5 Empirical And Molecular Formulae
Unit V The Mole Concept V 5 Empirical And Molecular Formulae
Percent Composition Griger Science
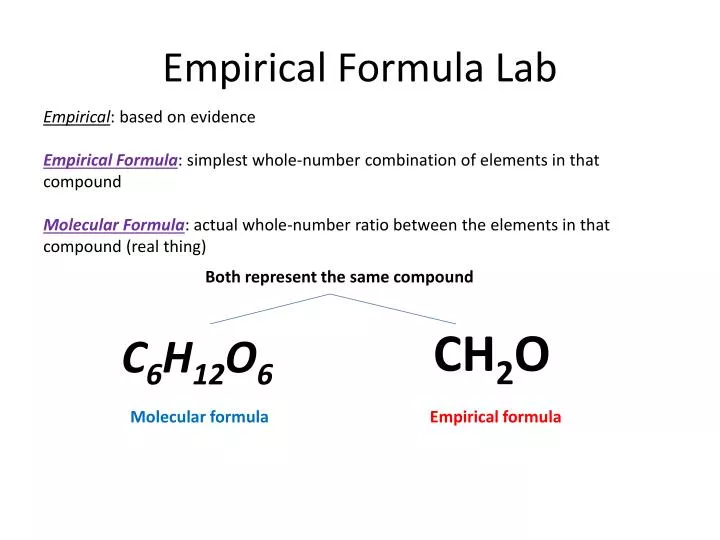 Ppt Empirical Formula Lab Powerpoint Presentation Free Download
Ppt Empirical Formula Lab Powerpoint Presentation Free Download
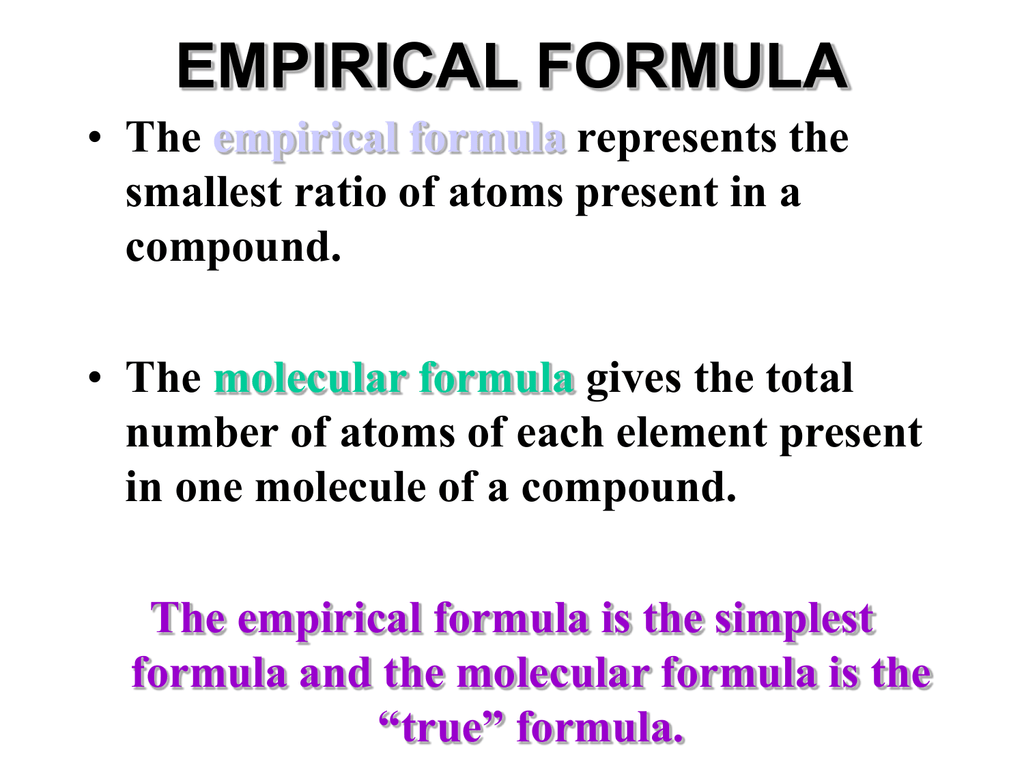
Posting Komentar
Posting Komentar