What Is The N In Pv Nrt
Solve for n pv nrt. A pv nrt problem.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcs9jd Vmbu0zytkmsgkp7kykaigomhvu Ceafilxaqfr1djfmfd Usqp Cau
The problems lie almost entirely in the units.

What is the n in pv nrt. R ideal gas constant. Cancel the common factor. Divide each term in by.
Cancel the common factor of. Ideal gas law solve for moles pv nrt solve for n. On the whole this is an easy equation to remember and use.
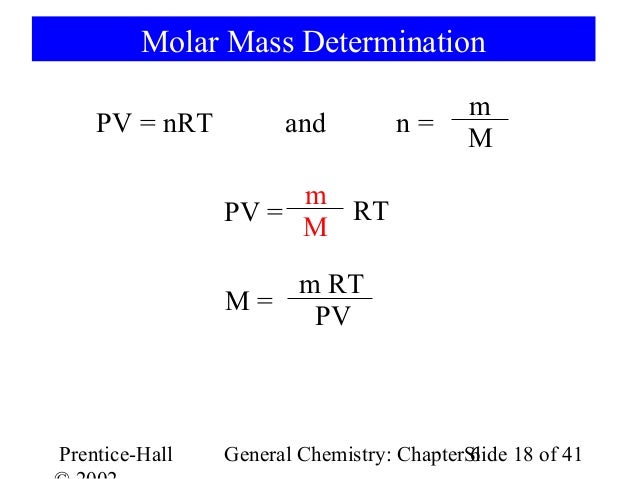
By convention pressure is converted into atmospheres atm volume into liters l and temperature into degrees kelvin k. It is almost 100 certain that you will be asked a determine molar mass question on your gas laws test. At stp a 5 00 l flask filled with air has a mass of 543 251 g.
Tap for more steps. Divide each term by and simplify. Exploring the various terms.
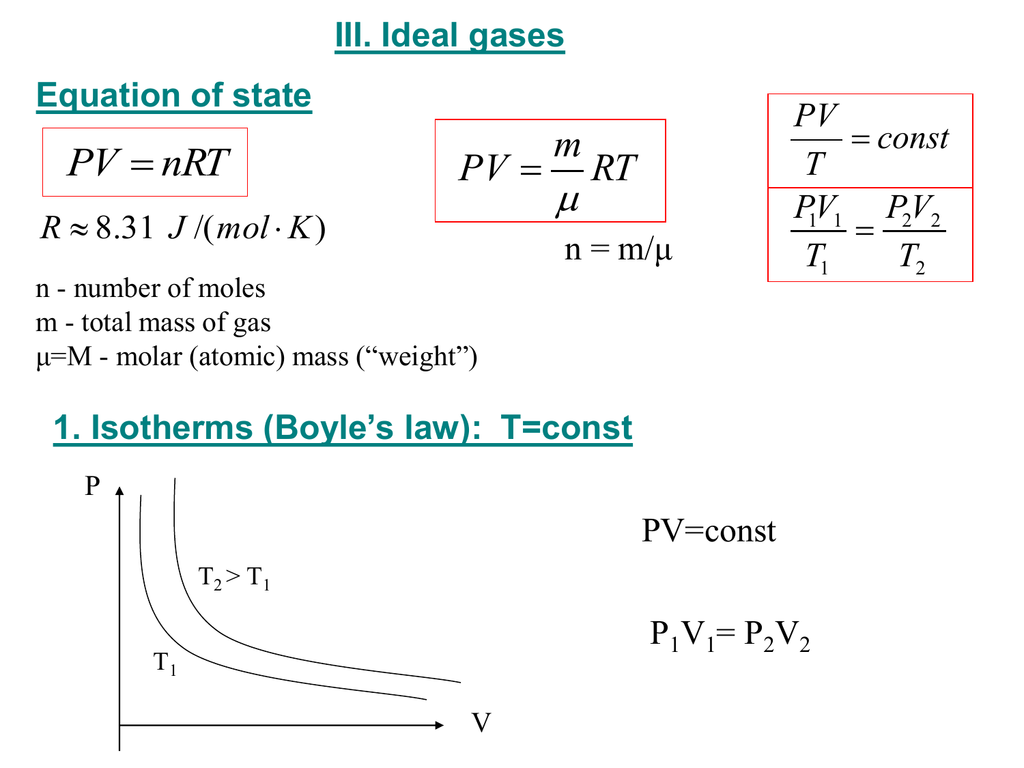
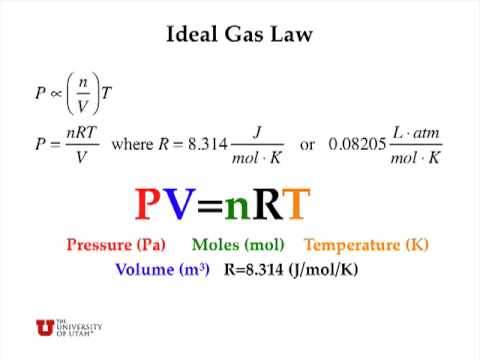
Ideal gas law for number of moles of gas n pv rt. In the equation pv nrt the term r stands for the universal gas constant. In chemistry the formula pv nrt is the state equation for a hypothetical ideal gas.
Make sure to use l atm and k. 3 divide grams by moles and there s your answer. Tap for more steps.
Tap for more steps. The air in the flask is replaced with. Ideal gases are defined as having molecules with negligible size with an average molar kinetic energy dependent only on temperature.
Examples on ideal gas law calculator with steps. The ideal gas law describes the behavior of an ideal sample of gas and how that behavior is related to the pressure p temperature t volume v and molarity n of the gas sample. Cancel the common factor of.
I am assuming below that you are working in strict si units as you will be if you are doing a uk based exam for example. Pv nrt 1 v 1 0 08206 273 15 v 22 41 l. What is the volume of 1 mole of an ideal gas at stp standard temperature and pressure 0 c 1 atm.
Tap for more steps. So the volume of an ideal gas is 22 41 l mol at stp. State the equation you plan to use and plug in the values.
This 22 4 l is probably the most remembered and least useful number in chemistry. Pv nrt where n is the number of moles of the gas and r is the ideal gas constant. N moles of gas.
Rewrite the equation as. Use pv nrt and solve for n. It states that pressure times volume equals the number of moles of gas molecules times temperature times the ideal gas constant.
The equation pv nrt is called the ideal gas law. Ideal gas law for r constant pv nt.
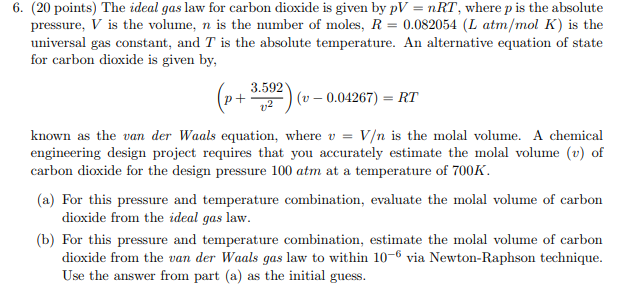 Solved 6 20 Points The Ideal Gas Law For Carbon Dioxid
Solved 6 20 Points The Ideal Gas Law For Carbon Dioxid
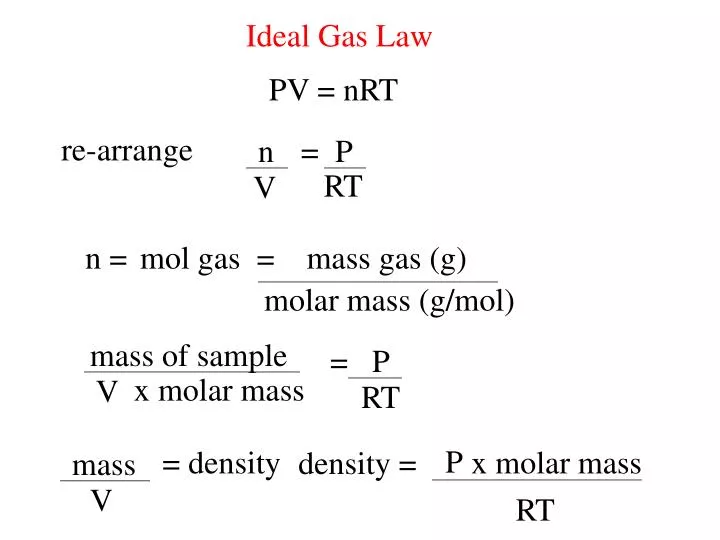 Ppt Ideal Gas Law Powerpoint Presentation Free Download Id
Ppt Ideal Gas Law Powerpoint Presentation Free Download Id
 Using The Ideal Gas Law To Calculate Number Of Moles Worked
Using The Ideal Gas Law To Calculate Number Of Moles Worked
 Deviations From Ideal Behavior 1 Mole Of Ideal Gas Pv Nrt N Pv
Deviations From Ideal Behavior 1 Mole Of Ideal Gas Pv Nrt N Pv
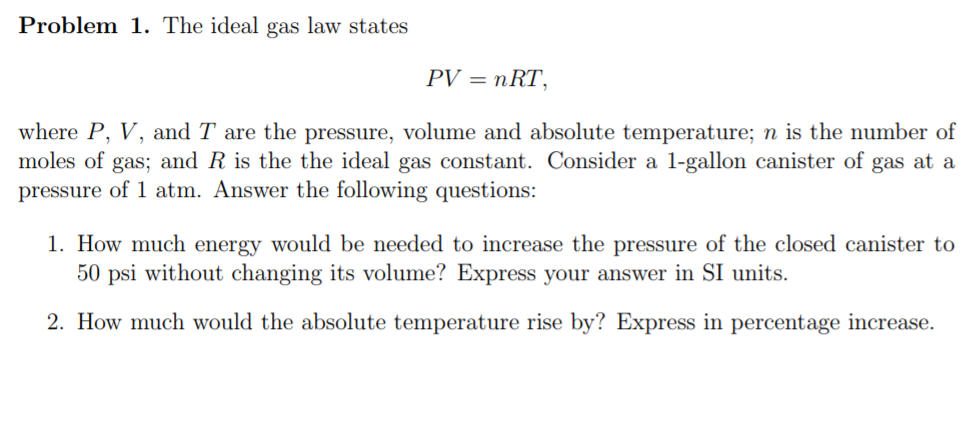 Solved Problem 1 The Ideal Gas Law States Pv Nrt Where
Solved Problem 1 The Ideal Gas Law States Pv Nrt Where
Ideal Gas Law Pv Nrt P Pressure Atm V Volume L Ppt Video
 The Ideal Gas Law Pv Nrt Relates Pressur Clutch Prep
The Ideal Gas Law Pv Nrt Relates Pressur Clutch Prep
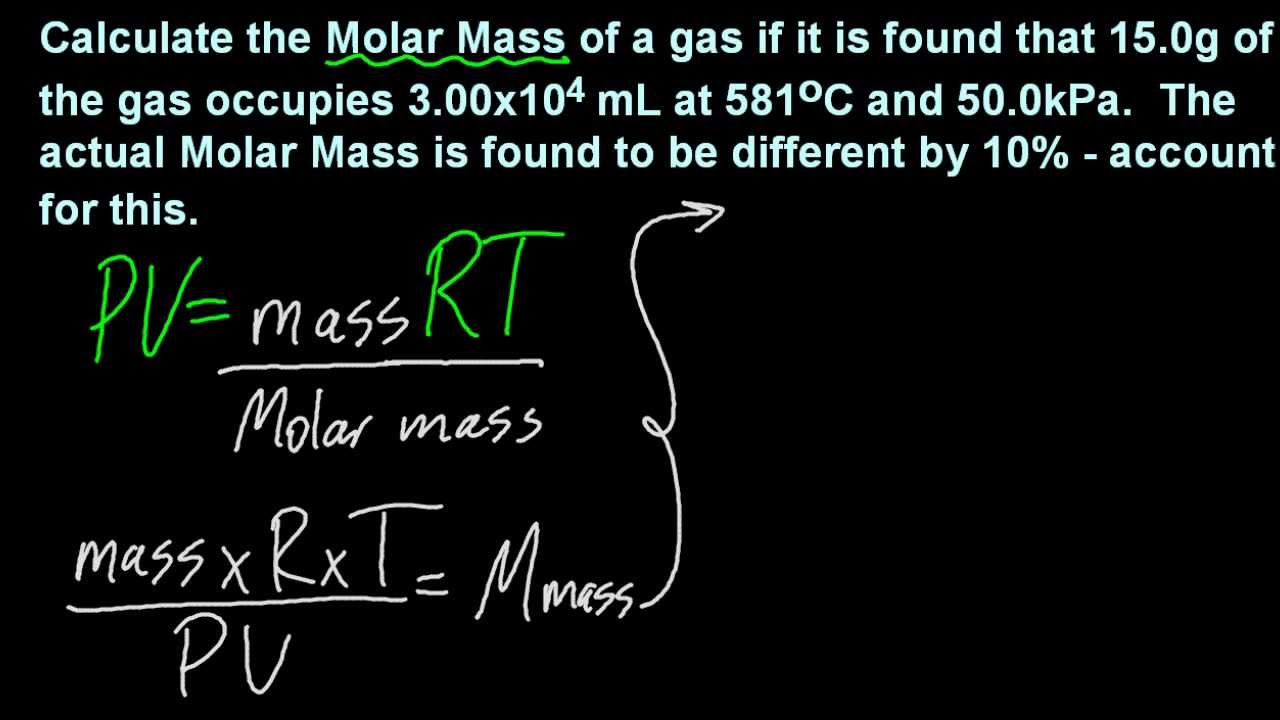 1 3 Solve Problems Using The Ideal Gas Equation Pv Nrt Sl Ib
1 3 Solve Problems Using The Ideal Gas Equation Pv Nrt Sl Ib
 Gas Equation Online Chemistry Tutorial That Deals With Chemistry
Gas Equation Online Chemistry Tutorial That Deals With Chemistry
 What Are The Gas Laws And Their Formulas By Chemistry Topics
What Are The Gas Laws And Their Formulas By Chemistry Topics
 Ppt Ideal Gas Law Powerpoint Presentation Free Download Id
Ppt Ideal Gas Law Powerpoint Presentation Free Download Id
 Pv Nrt Use The Ideal Gas Law Youtube
Pv Nrt Use The Ideal Gas Law Youtube
 The Ideal Gas Law Co Ppt Download
The Ideal Gas Law Co Ppt Download
 Solved The Ideal Gas Law States That Pv Nrt Where P Is
Solved The Ideal Gas Law States That Pv Nrt Where P Is
 12 The Gaseous State Of Matter Ppt Download
12 The Gaseous State Of Matter Ppt Download
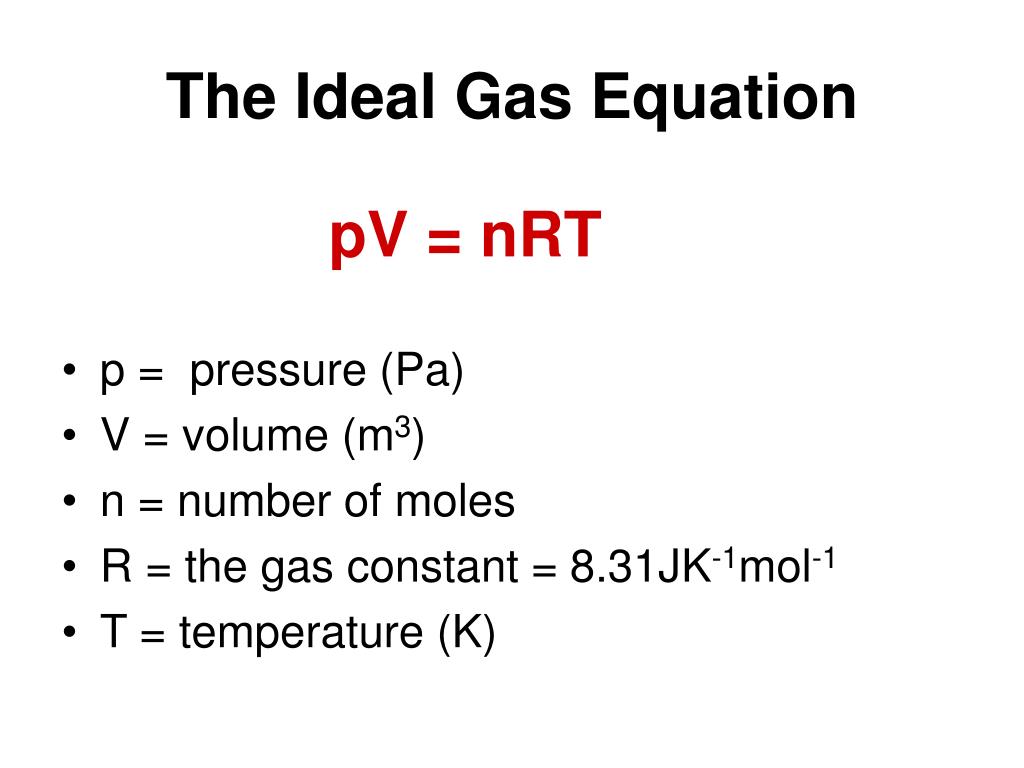 Ppt The Ideal Gas Equation Powerpoint Presentation Free
Ppt The Ideal Gas Equation Powerpoint Presentation Free
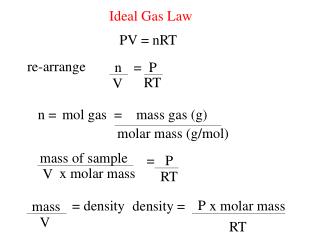 Ppt Ideal Gas Law Powerpoint Presentation Free Download Id
Ppt Ideal Gas Law Powerpoint Presentation Free Download Id
 What Is The Graphical Representation Between N And T In The Ideal
What Is The Graphical Representation Between N And T In The Ideal
 Ideal Gas Law Chemistry Hybrid
Ideal Gas Law Chemistry Hybrid
 What Is N In The Equation Of The Ideal Gas Law Socratic
What Is N In The Equation Of The Ideal Gas Law Socratic
 Finding Pv In Pv Nrt For Molecular Hydrogen Chemistry Stack Exchange
Finding Pv In Pv Nrt For Molecular Hydrogen Chemistry Stack Exchange
 General Chemistry Ideal Gas Law Pv Nrt Example 2 Youtube
General Chemistry Ideal Gas Law Pv Nrt Example 2 Youtube
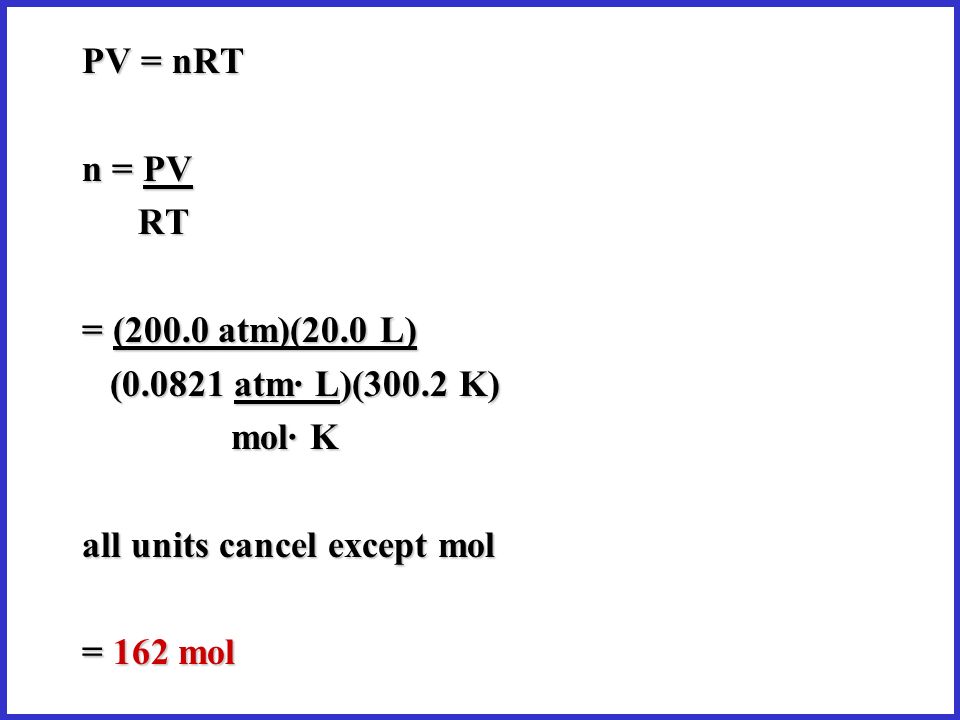 The Kinetic Theory Pressure Gas Laws Ppt Video Online Download
The Kinetic Theory Pressure Gas Laws Ppt Video Online Download
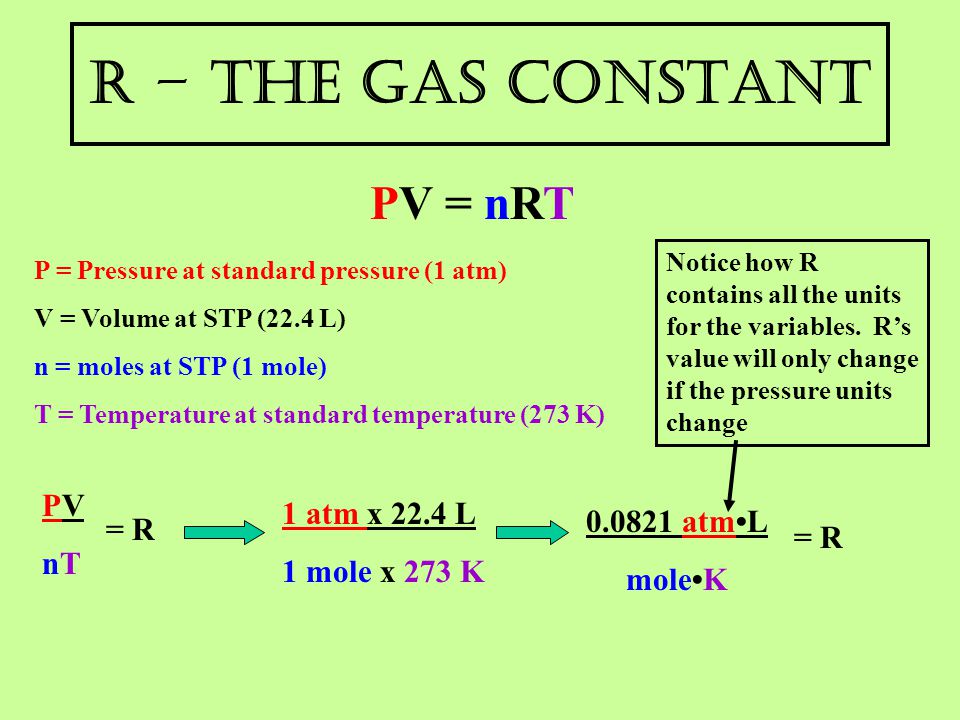 The Gas Laws Pv Nrt Ppt Video Online Download
The Gas Laws Pv Nrt Ppt Video Online Download
 Nkt Pv Nrt Pv Pa Pressure P M Volume V Moles N Particles
Nkt Pv Nrt Pv Pa Pressure P M Volume V Moles N Particles
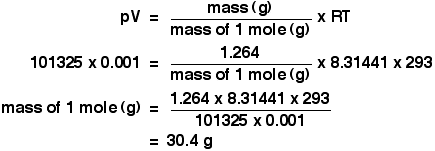 Ideal Gases And The Ideal Gas Law Pv Nrt
Ideal Gases And The Ideal Gas Law Pv Nrt
Pv Nrt The Ideal Gas Law Youtube
 Pv Nrt The Ideal Gas Law Youtube
Pv Nrt The Ideal Gas Law Youtube
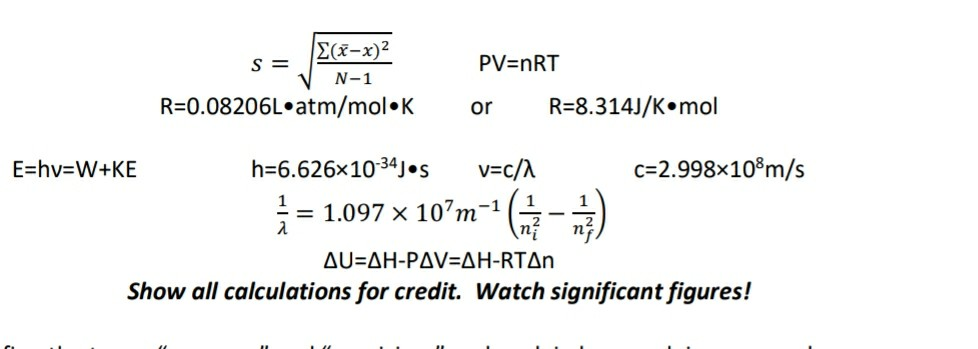 Solved Pv Nrt N 1 R 0 08206l Atm Mol K Or R 8 314j K Mol
Solved Pv Nrt N 1 R 0 08206l Atm Mol K Or R 8 314j K Mol
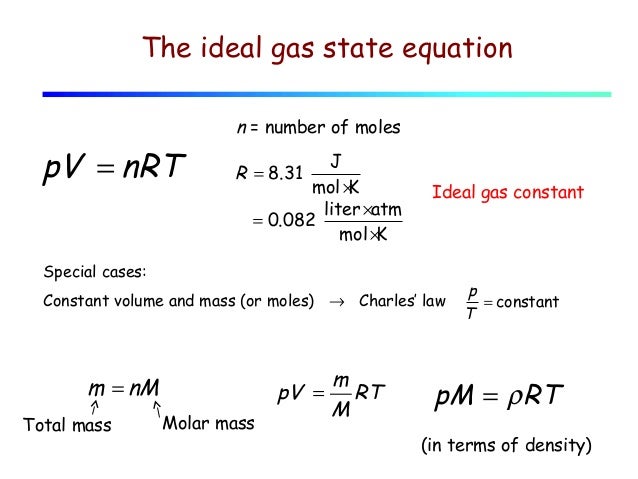 Lecture 13 Ideal Gas Kinetic Model Of A Gas
Lecture 13 Ideal Gas Kinetic Model Of A Gas
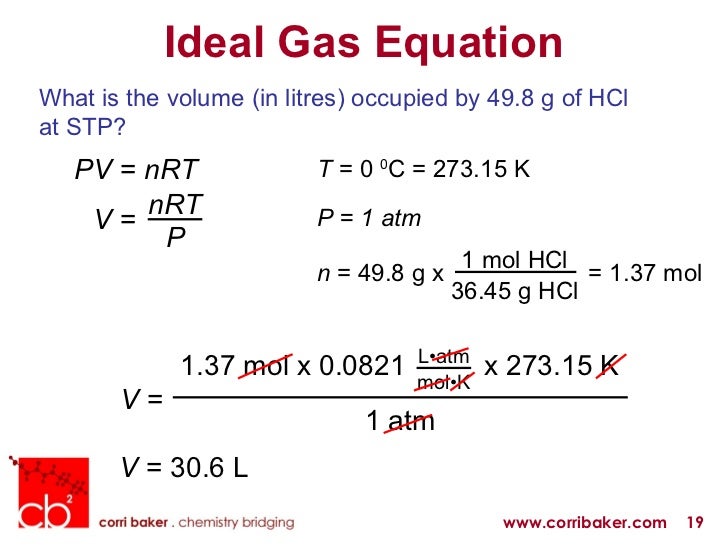
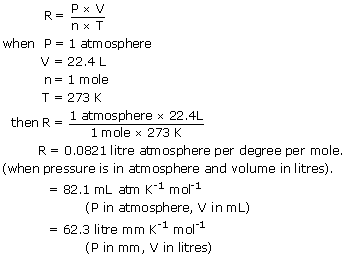



Posting Komentar
Posting Komentar