Real Gas Vs Ideal Gas Pressure
The deviations from ideal gas behaviour can be illustrated as follows. Real gases are composed of atoms or molecules resulting in their volume.
 Density Blue Line Ideal Gas Density At Pressure P D P P Rt
Density Blue Line Ideal Gas Density At Pressure P D P P Rt
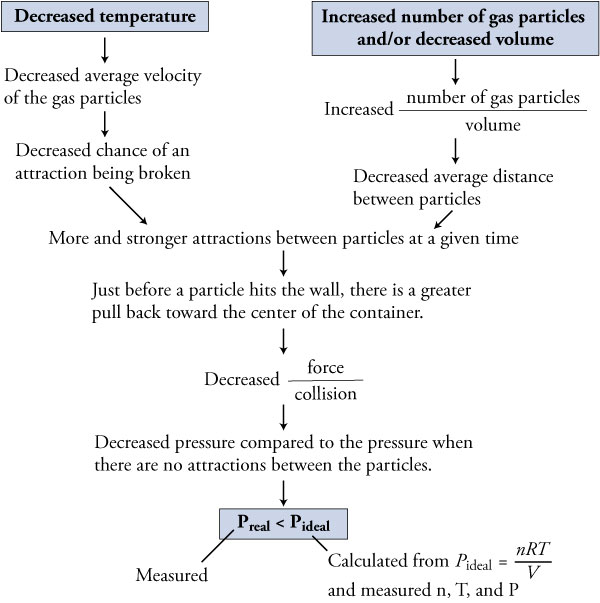
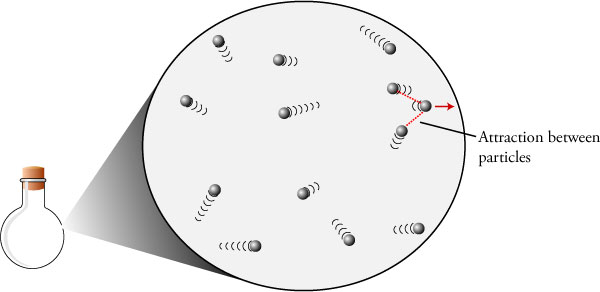
The pressure is much greater in ideal gas as compared to the pressure of a real gas since the particles do not have the attractive forces that enable the molecules to hold back when they will collide at an impact.

Real gas vs ideal gas pressure. Real gases have inter molecular attraction and when expands the molecules have to spend more kinetic energy to overcome inter molecular attraction compare to an ideal gas. They also follow gas laws. An ideal gas is a gaseous compound that does not exist in reality but is a hypothetical gas.
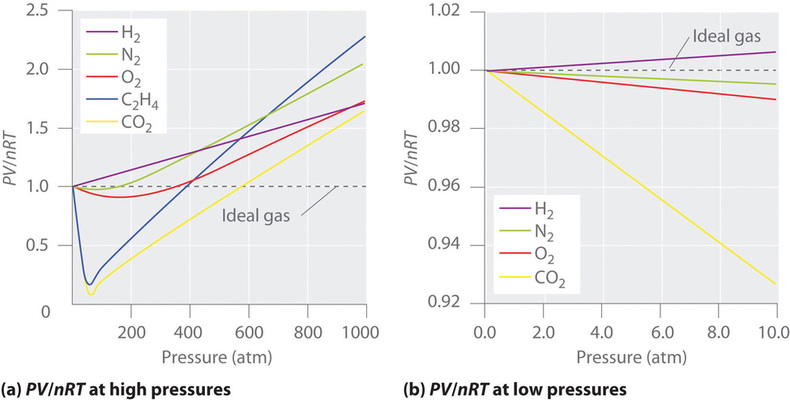
For a real gas like nitrogen notice how the compression factor tends to increase with pressure. For an ideal gas the compression factor would be 1 over the whole pressure range. In an ideal gas if we compress the gas by increasing p the density ρ must increase as well so as to keep z 1.
In addition ideal gases come to the real state at very low temperatures. However some gaseous compounds show approximately similar behavior to that of ideal gases at a specific temperature and pressure conditions. The difference between ideal gas and real gas is real gas has real volume while ideal gas does not.
Real gases pass through porous plug from higher pressure to lower pressure within the insulated enclosure there occurs a change of temperature. Therefore they collide with less force. Then compared to the space we cannot ignore the size of the molecule.
They nearly obey ideal gas equation at higher temperatures and very low pressures. Hence particles collide with less energy. The pressure of an ideal gas is much greater than that of a real gas since its particles lack the attractive forces which hold the particles back when they collide.
Two graphs of the compressibility factor z vs. Pressure at 273 k. For a real gas z therefore gives us a measure of how much the gas deviates from ideal gas behavior.
A real gas varies from the ideal condition at very high pressures. Real gases these are a type of nonhypothetical gas that have mass and volume. A real gas is a gaseous compound that really exists.
This is because when a very high pressure is applied the volume where the gas is filled becomes very smaller. The value of the compression factor is too high at high pressures for a real gas. Real gases do not obey ideal gas equation under all conditions.
The associated molecules have interactions and space. However they show deviations from ideality at low temperatures and high pressures.
 Ideal Vs Real Gases No Gas Is Ideal As The Temperature Of A Gas
Ideal Vs Real Gases No Gas Is Ideal As The Temperature Of A Gas
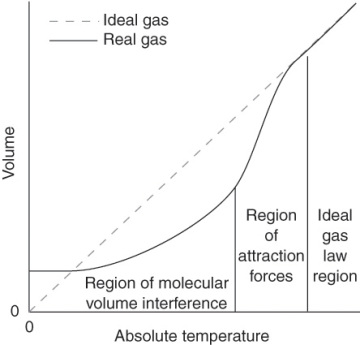 4 5 Real Gases Gas Laws Pressure Volume And Temperature
4 5 Real Gases Gas Laws Pressure Volume And Temperature
 Behaviour Of Real Gases Deviation From Ideal Gas Behaviour The
Behaviour Of Real Gases Deviation From Ideal Gas Behaviour The
 8 6 Non Ideal Gas Behavior General College Chemistry I
8 6 Non Ideal Gas Behavior General College Chemistry I
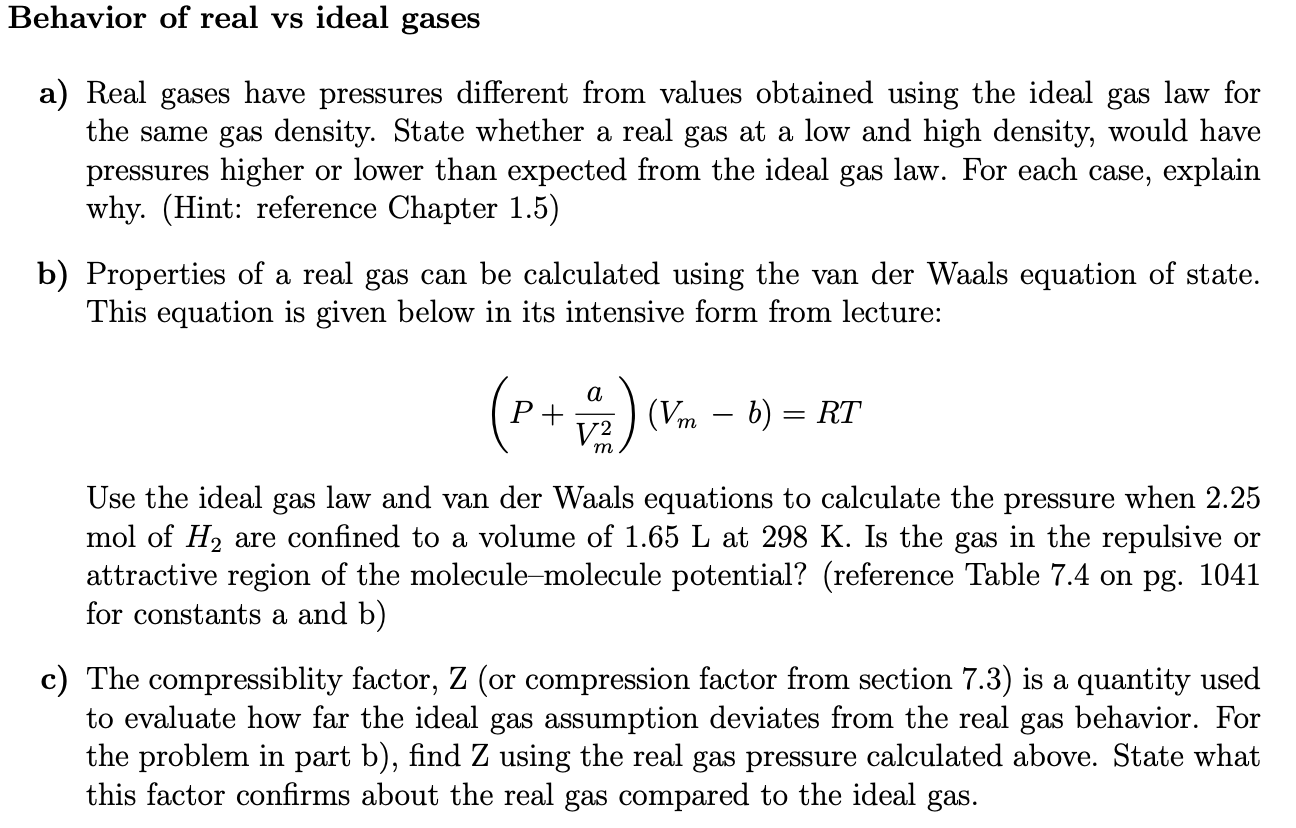 Solved Behavior Of Real Vs Ideal Gases A Real Gases Have
Solved Behavior Of Real Vs Ideal Gases A Real Gases Have
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqtkc Gyl1p2tqex6qp Ohddbww3jsg16ffaydfvt3agxnejflg Usqp Cau
Deviations From Ideal Gas Law Behavior
 8 6 Non Ideal Gas Behavior General College Chemistry I
8 6 Non Ideal Gas Behavior General College Chemistry I
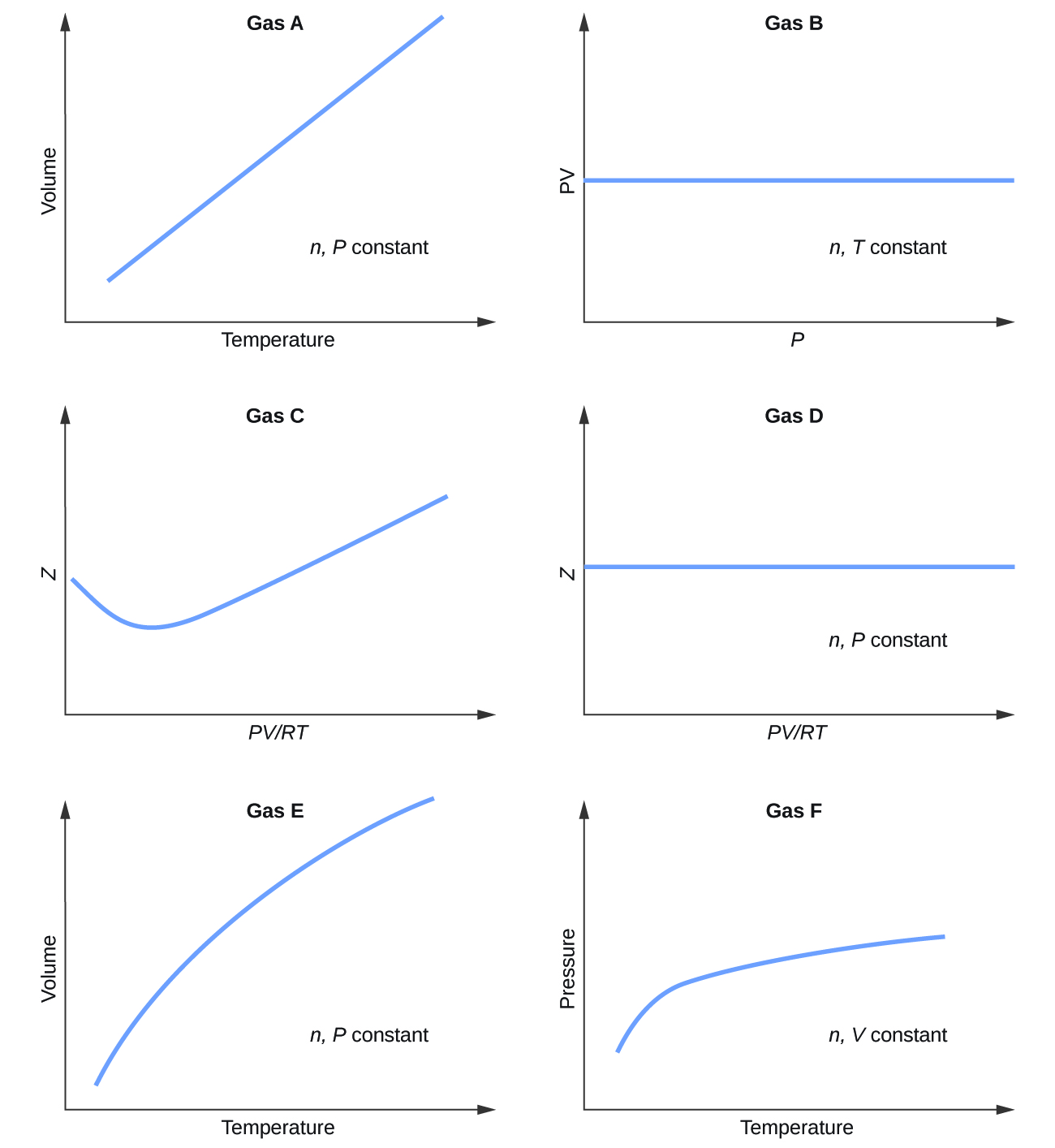 9 6 Non Ideal Gas Behavior Chemistry
9 6 Non Ideal Gas Behavior Chemistry
 Behaviour Of Real Gases Deviation From Real Behaviour Videos
Behaviour Of Real Gases Deviation From Real Behaviour Videos
 Pressure Versus Volume Graph For Real Gas And Are Shown In Figure
Pressure Versus Volume Graph For Real Gas And Are Shown In Figure
 8 6 Non Ideal Gas Behavior General College Chemistry I
8 6 Non Ideal Gas Behavior General College Chemistry I
 10 9 Real Gases Deviations From Ideal Behavior Chemistry
10 9 Real Gases Deviations From Ideal Behavior Chemistry
 Behavior Of Real Gases Deviations From Ideal Gas Behavior
Behavior Of Real Gases Deviations From Ideal Gas Behavior
Pressure Versus Volume Graph For A Real Gas And An Ideal Gas Are
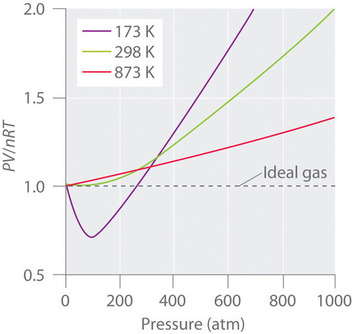 10 9 Real Gases Deviations From Ideal Behavior Chemistry
10 9 Real Gases Deviations From Ideal Behavior Chemistry
 Ideal Gases Vs Real Gases Schoolworkhelper
Ideal Gases Vs Real Gases Schoolworkhelper
 When Do Real Gases Act Like Ideal Gases Youtube
When Do Real Gases Act Like Ideal Gases Youtube
 Pressure Vs Volume Plot For Real And Ideal Gasses Chemistry
Pressure Vs Volume Plot For Real And Ideal Gasses Chemistry
 Why Is The Pressure Exerted By Ideal Gas On The Walls Of The
Why Is The Pressure Exerted By Ideal Gas On The Walls Of The
 Why Do Real Gases React Like Ideal Gases Only In High Temperature
Why Do Real Gases React Like Ideal Gases Only In High Temperature
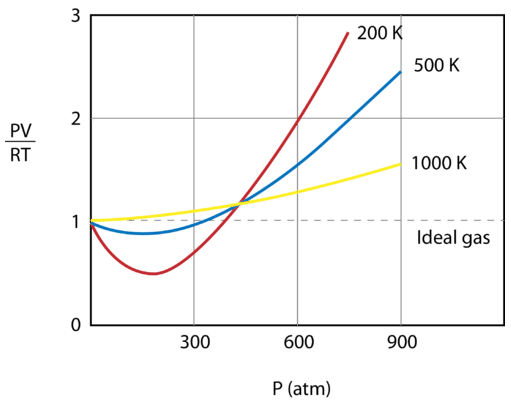 14 11 Real And Ideal Gases Chemistry Libretexts
14 11 Real And Ideal Gases Chemistry Libretexts
 Real Vs Ideal Gas Behavior Video Khan Academy
Real Vs Ideal Gas Behavior Video Khan Academy
Difference Between Real And Ideal Gas Definition Specific
 Motion Of Particles Ideal Gases Siyavula
Motion Of Particles Ideal Gases Siyavula
![]() 5 9 Behaviour Of Real Gases Deviation From Ideal Gas Behaviour
5 9 Behaviour Of Real Gases Deviation From Ideal Gas Behaviour
 Real Gases Using The Van Der Waals Equation Video Lesson
Real Gases Using The Van Der Waals Equation Video Lesson

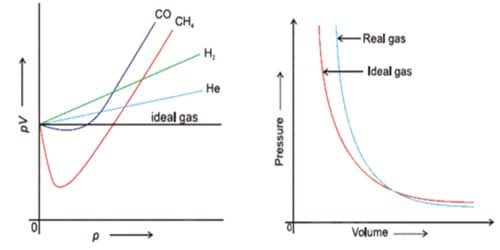 Explain The Causes Of Deviation Of Real Gases From Ideal Behavior
Explain The Causes Of Deviation Of Real Gases From Ideal Behavior
 Comparison Of R134a Property Using Ideal Gas And Real Gas Laws
Comparison Of R134a Property Using Ideal Gas And Real Gas Laws
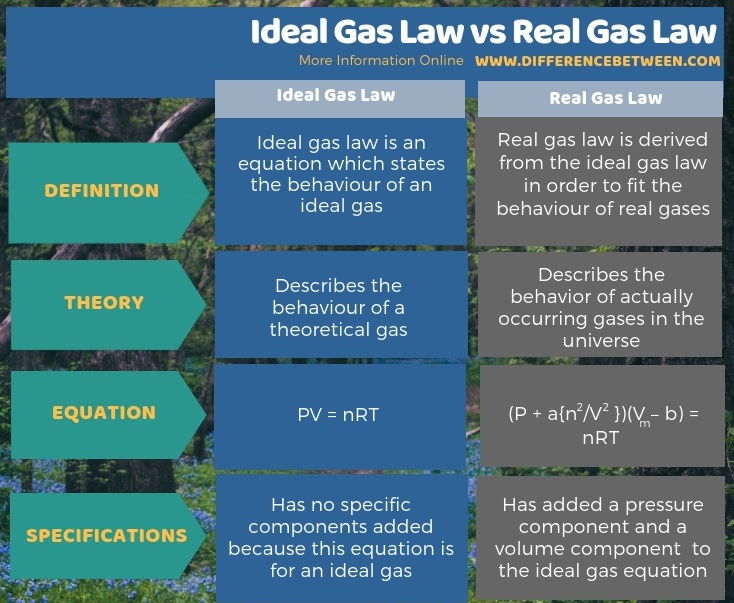 Difference Between Ideal Gas Law And Real Gas Law Compare The
Difference Between Ideal Gas Law And Real Gas Law Compare The
 Non Ideal Behavior Of Gases Article Khan Academy
Non Ideal Behavior Of Gases Article Khan Academy
 Ideal Gas An Overview Sciencedirect Topics
Ideal Gas An Overview Sciencedirect Topics
 Motion Of Particles Ideal Gases Siyavula
Motion Of Particles Ideal Gases Siyavula


Posting Komentar
Posting Komentar