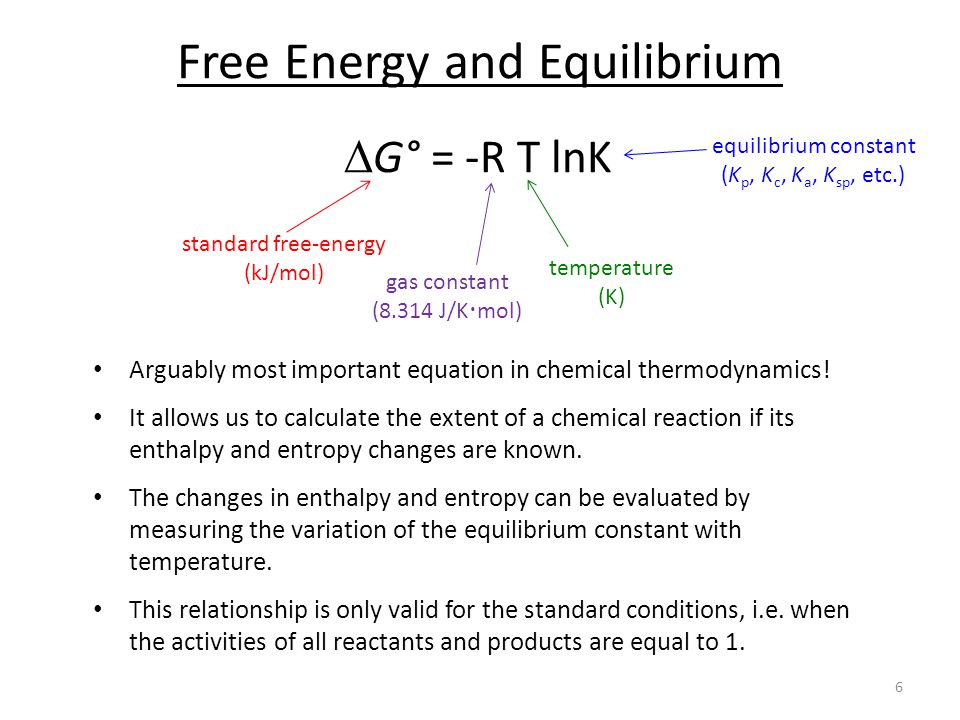
Standard Gibbs Free Energy Formula
δh δg s definitions of standard states. We can use the relationship between δg and the equilibrium constant k to obtain a relationship between e cell and k.
Gibbs Energy And Reaction Diagrams Chemical Kinetics Flashcards
The free energy of a system is the sum of its enthalpy h plus the product of the temperature kelvin and the entropy s of the system.

Standard gibbs free energy formula. Recall that for a general reaction of the type aa bb cc dd the standard free energy change and the equilibrium constant are related by the following equation. The term standard state is used to describe a reference state for substances and is a help in thermodynamical calculations as enthalpy entropy and gibbs free energy calculations. The change in free energy δ g is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system.
How the second law of thermodynamics helps us determine whether a process will be spontaneous and using changes in gibbs free energy to predict whether a reaction will be spontaneous in the forward or reverse direction or whether it is at equilibrium. The gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions. Gibbs free energy g the energy associated with a chemical reaction that can be used to do work.
G h ts. δg rtlnk. If i combust one mole of hydrogen gas in mole of oxygen gas to form 1 mole of liquid water at 298 2 k then the gibbs free energy of formation is 237 2 kj mol 1.
δh change in enthalpy. δg δg ƒ x kj mol 1. The table below lists the standard gibbs function.
δ g can predict the direction of the chemical reaction under two conditions. The gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. The maximum work done is the amount of energy produced given by the decrease in the thermodynamic property called gibbs free energy.
The superscript degree symbol indicates that substances are in their standard states. The gibbs free energy measured in joules in si is the maximum amount of non expansion work that can be extracted from a thermodynamically closed system can exchange heat and work with its surroundings but not matter. For a gas the standard state is as a pure gaseous substance as a.
Gibbs free energy denoted g combines enthalpy and entropy into a single value. Gibbs free energy formula is given below. The standard gibbs free energy of formation of a compound is the change of gibbs free energy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states the most stable form of the element at 1 bar of pressure and the specified temperature usually 298 15 k or 25 c.
H 2 g o 2 g h 2 o l δg ƒ 237 2 kj mol 1.
 Topic 10 Free Energy Equilibrium Constant 02 Studocu
Topic 10 Free Energy Equilibrium Constant 02 Studocu
 Ap Chemistry Non Standard Gibbs Free Energy Worksheet Review
Ap Chemistry Non Standard Gibbs Free Energy Worksheet Review
Gibbs Free Energy And The Spontaneity Of Chemical Reactions
How Much Energy Is Released In Atp Hydrolysis
How Is Gibbs Free Energy Related To Enthalpy And Entropy Socratic
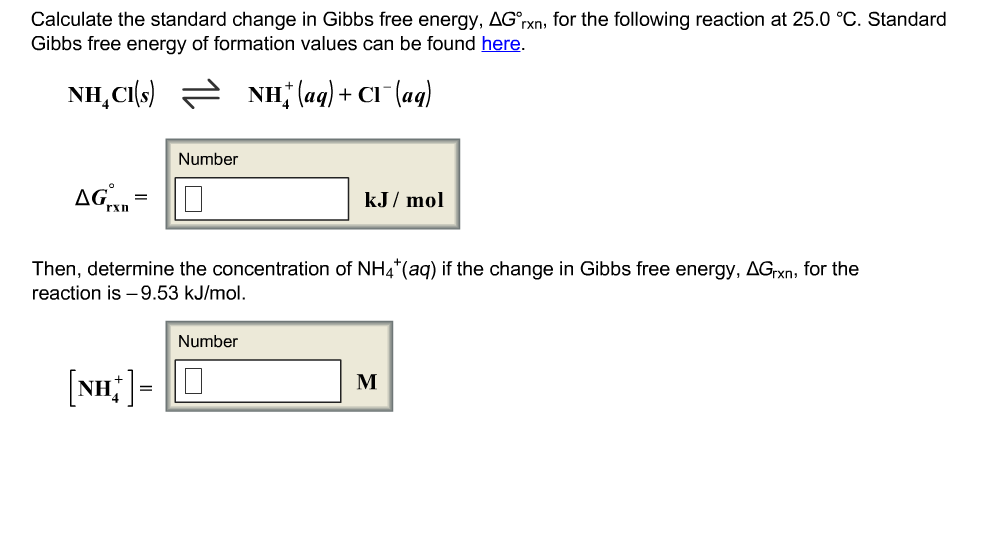
 Oneclass Calculate The Standard Change In Gibbs Free Energy Ga
Oneclass Calculate The Standard Change In Gibbs Free Energy Ga
 Calculate Standard Gibbs Free Energy Energy Etfs
Calculate Standard Gibbs Free Energy Energy Etfs
 17 1 Equilibrium And Gibbs Free Energy Hl Youtube
17 1 Equilibrium And Gibbs Free Energy Hl Youtube
 Gibbs Free Energy Of Formation An Overview Sciencedirect Topics
Gibbs Free Energy Of Formation An Overview Sciencedirect Topics
 Review Standard Gibbs Free Energy Go Ho T So Go
Review Standard Gibbs Free Energy Go Ho T So Go
 Chem 2 Std Free Energy Of Formation Vii
Chem 2 Std Free Energy Of Formation Vii
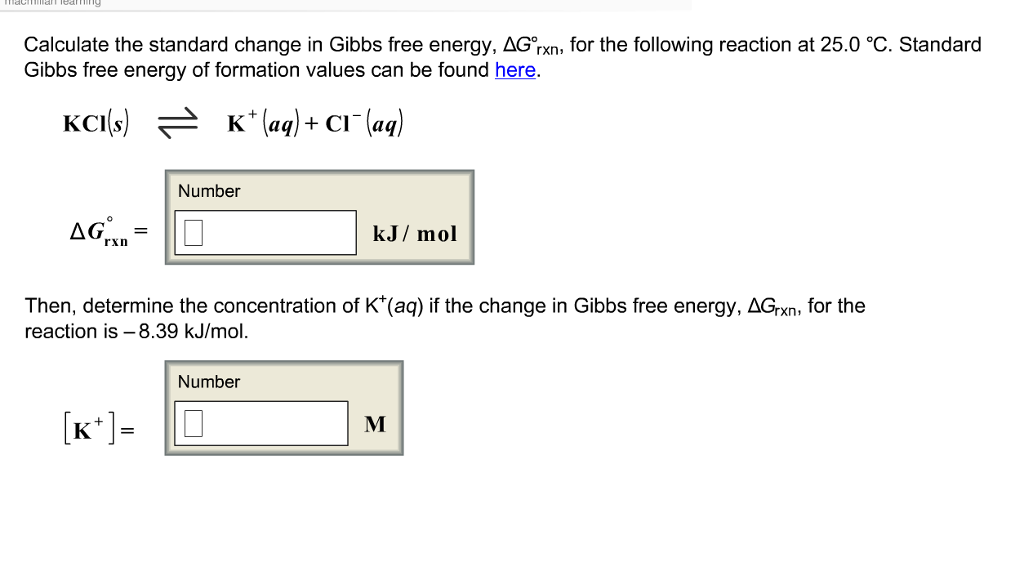
 Solved Calculate The Standard Change In Gibbs Free Energy
Solved Calculate The Standard Change In Gibbs Free Energy
 Solved Calculate The Standard Change In Gibbs Free Energy
Solved Calculate The Standard Change In Gibbs Free Energy
 Reaction Quotient And Gibbs Free Energy At The Start Of A Reaction
Reaction Quotient And Gibbs Free Energy At The Start Of A Reaction
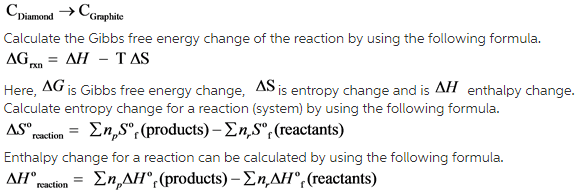 What Is The Standard Gibbs Free Energy For The Transformation Of
What Is The Standard Gibbs Free Energy For The Transformation Of
Free Energy Changes In Biochemical Systems
 Temperature In The Gibbs Free Energy Equation Chemistry Stack
Temperature In The Gibbs Free Energy Equation Chemistry Stack
 Standard Free Energy Changes Introduction To Chemistry
Standard Free Energy Changes Introduction To Chemistry
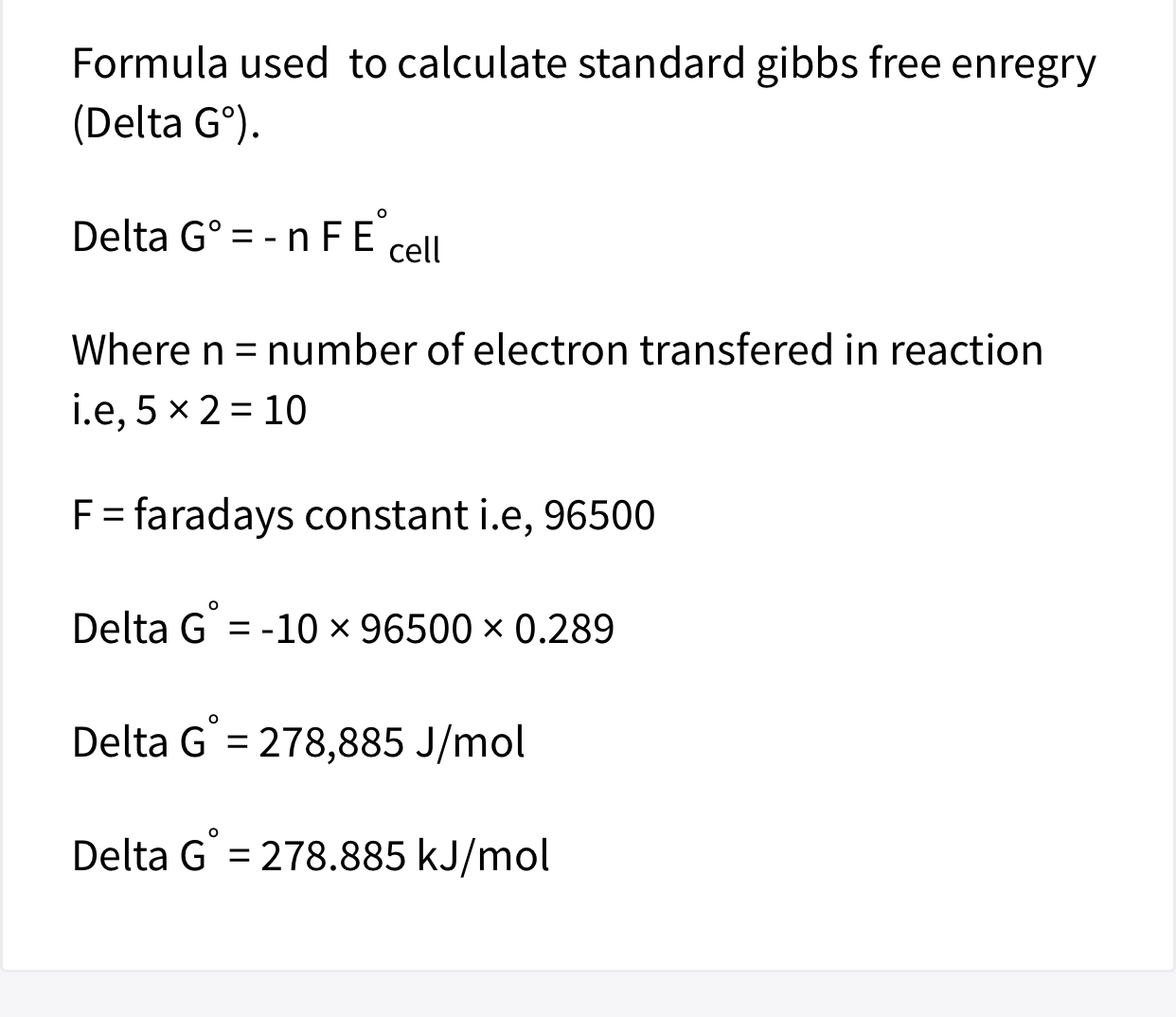 Answered 6 What Is The Standard Gibbs Free Bartleby
Answered 6 What Is The Standard Gibbs Free Bartleby
 Calculate The Standard Change In Gibbs Fre Clutch Prep
Calculate The Standard Change In Gibbs Fre Clutch Prep
 Temperature In The Gibbs Free Energy Equation Chemistry Stack
Temperature In The Gibbs Free Energy Equation Chemistry Stack
Helmholtz And Gibbs Free Energies
 Calculate The Standard Gibbs Free Energy Change Dgo For The
Calculate The Standard Gibbs Free Energy Change Dgo For The
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsox Hhyvknjanf1tnseoz72om4nntckufqvm Ohk5acd Nmhoz Usqp Cau
 Calculate The Standard Change In Gibbs Free Energy For The
Calculate The Standard Change In Gibbs Free Energy For The
 Entropy Standard Gibbs Free Energy Of Reaction And Chemical
Entropy Standard Gibbs Free Energy Of Reaction And Chemical
 Standard Gibbs Free Energy Change And Standard Enthtlyy Changes Of
Standard Gibbs Free Energy Change And Standard Enthtlyy Changes Of
 Getting Gibbs Energy As A Function Of Temperature
Getting Gibbs Energy As A Function Of Temperature
Department Of Chemistry And Biochemistry Chem 4520 Metabolic
 Gibbs Free Energy Example Video Khan Academy
Gibbs Free Energy Example Video Khan Academy
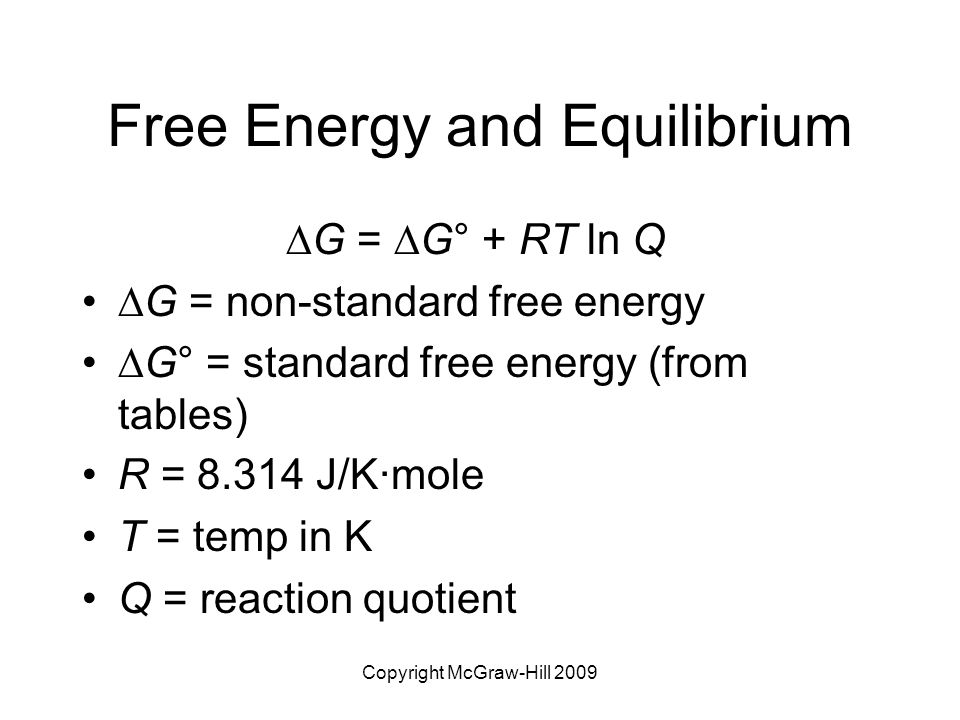 Chapter 18 Entropy Free Energy And Equilibrium Ppt Video Online
Chapter 18 Entropy Free Energy And Equilibrium Ppt Video Online
Department Of Chemistry And Biochemistry Chem 4520 Metabolic
 Solved Calculate The Standard Change In Gibbs Free Energy
Solved Calculate The Standard Change In Gibbs Free Energy




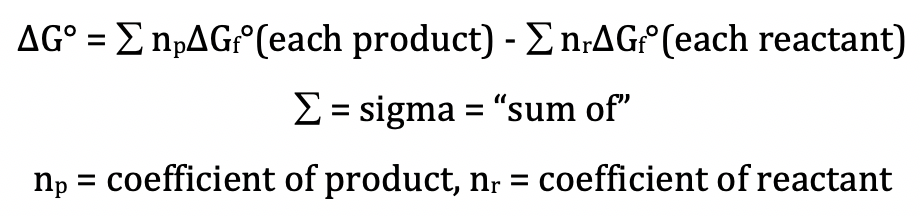
Posting Komentar
Posting Komentar