Ideal Gas Law Vs Real Gas
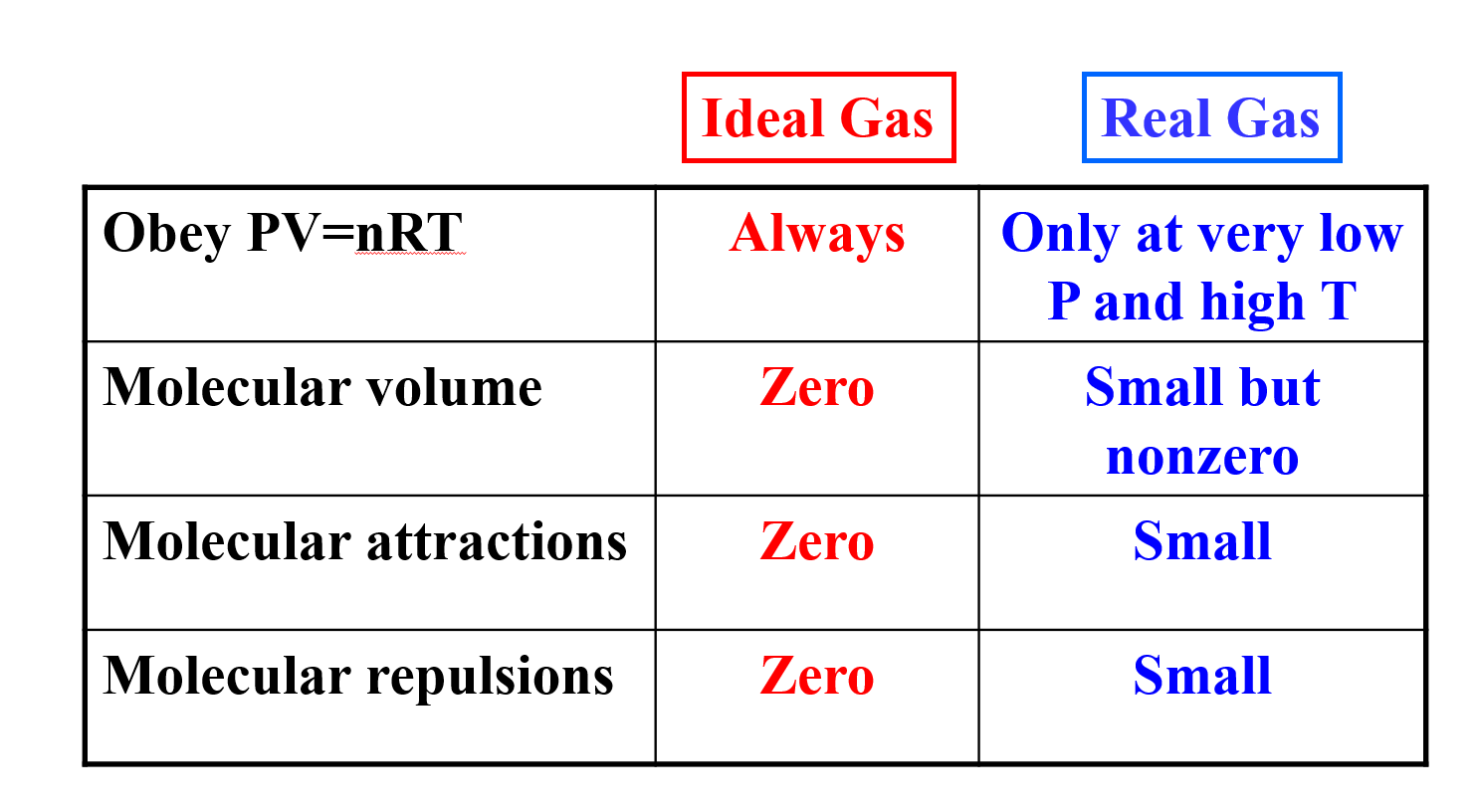
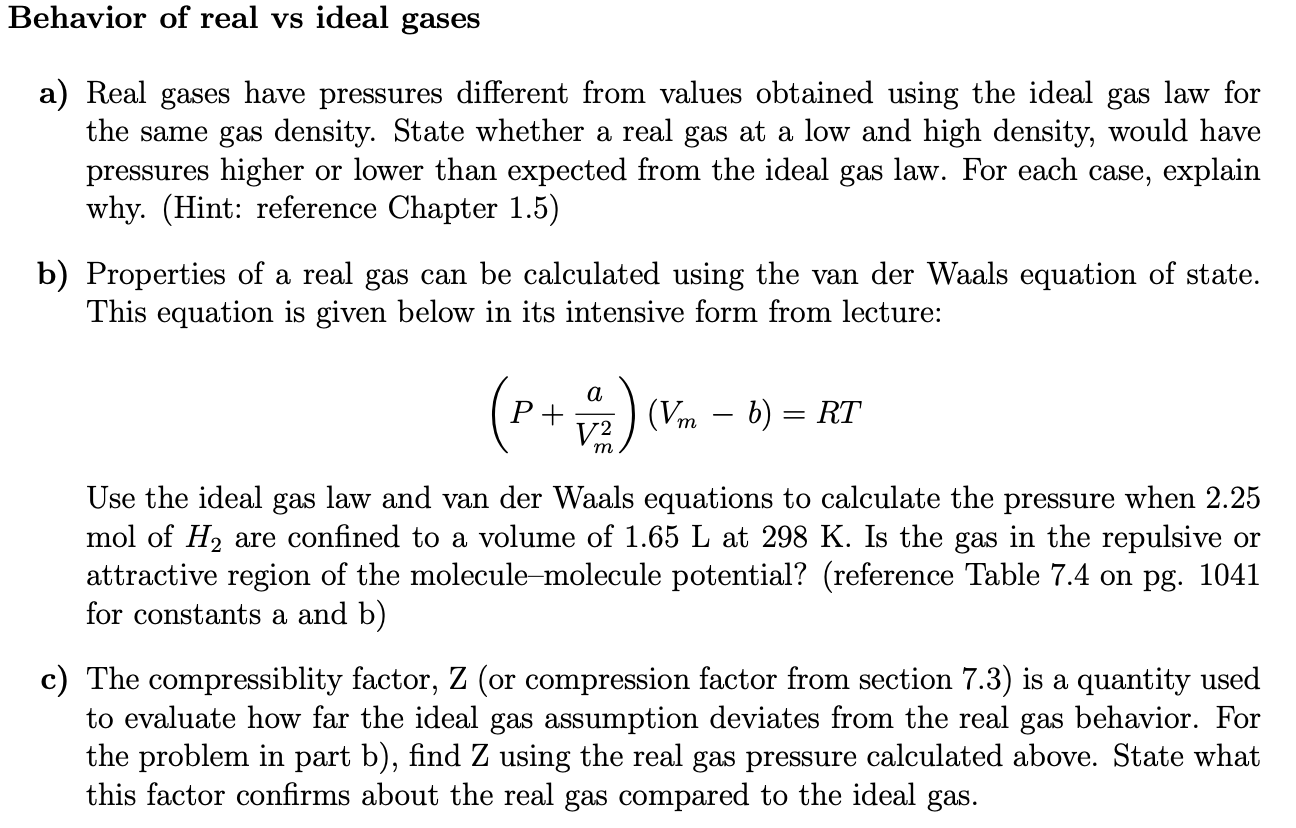
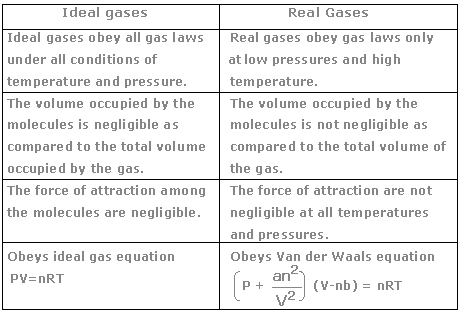
The ideal gas law may be expressed as. Therefore for ideal or perfect gases the compressibility factor z 1.
 Ideal Vs Real Gases No Gas Is Ideal As The Temperature Of A Gas
Ideal Vs Real Gases No Gas Is Ideal As The Temperature Of A Gas
2 ideal gas has no mass whereas real gas has mass.
/149263439-56a12f093df78cf7726836ed.jpg)
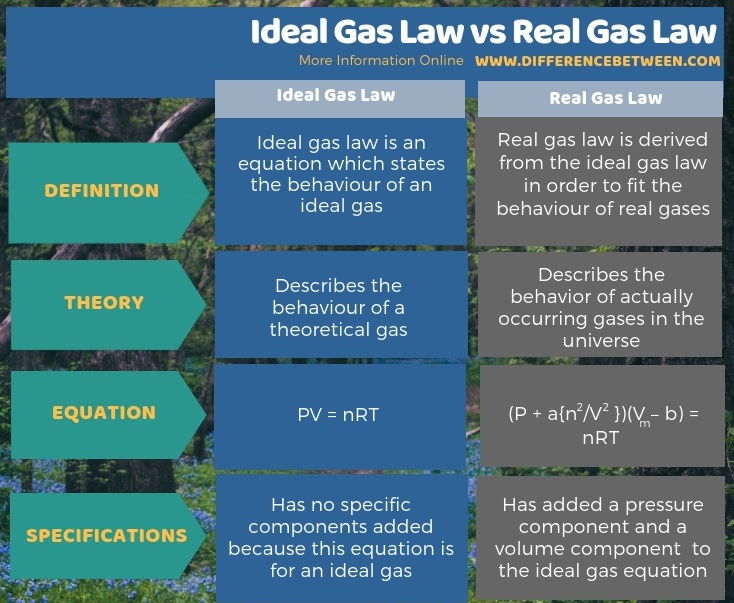
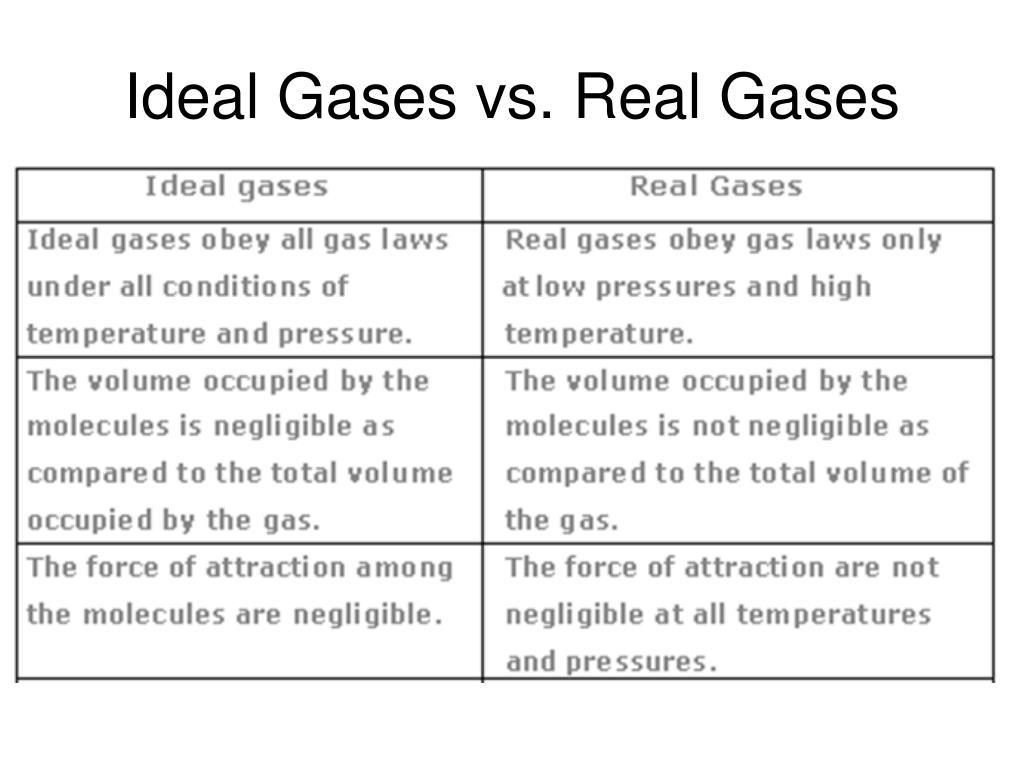
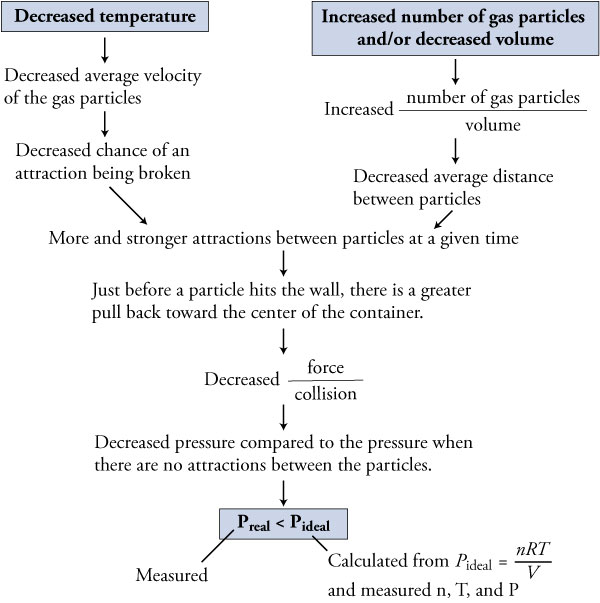
Ideal gas law vs real gas. An ideal gas is a theoretical gas which perfectly fits into the equation pv nrt. But for real gases z 1. 1 ideal gas has no definite volume while real gas has definite volume.
Real gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. Ideal gases obtain no volume unlike real gases which obtain small volumes. But from ideal gas equation.
They also follow gas laws. Ideal gases do not have intermolecular forces and the gas molecules considered as point particles. 3 collision of ideal gas particles is elastic while non elastic for real gas.
However some gaseous compounds show approximately similar behavior to that of ideal gases at a specific temperature and pressure conditions. When the gas becomes massive and voluminous it deviates from its ideal behaviour. Ideal gases cannot be found in reality.
An ideal gas is a gaseous compound that does not exist in reality but is a hypothetical gas. A real gas is a gaseous compound that really exists. The key difference between ideal gas law and real gas law is that ideal gas law describes the behaviour of a theoretical gas whereas real gas law describes the behaviour of actually occurring gases in the universe.
Further they have intermolecular forces. The ideal gas law is one of the equations of state. The difference between ideal gas and real gas is real gas has real volume while ideal gas does not.
Real gases these are a type of nonhypothetical gas that have mass and volume. And in real gases in order to assume they re like an ideal gas we assume this is very limited or that we can assume they re not happening. 4 no energy involved during collision of particles in ideal gas.
For ideal gases and for the application of the ideal gas law we assume that there are no intermolecular interactions or if there are that it s very. An ideal gases mass can be disregarded in the equation because it has none. We assume an ideal gas has none of them.
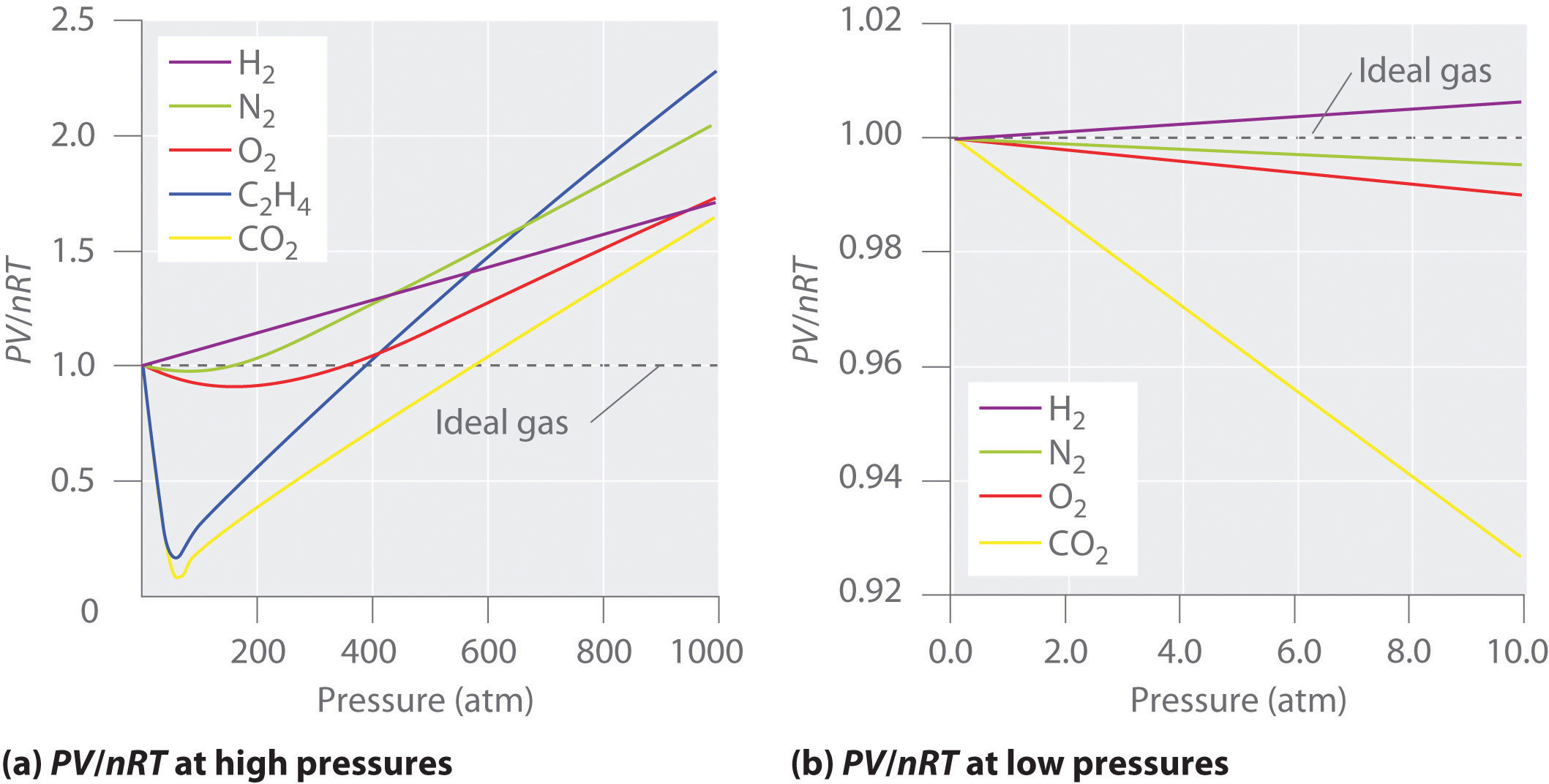
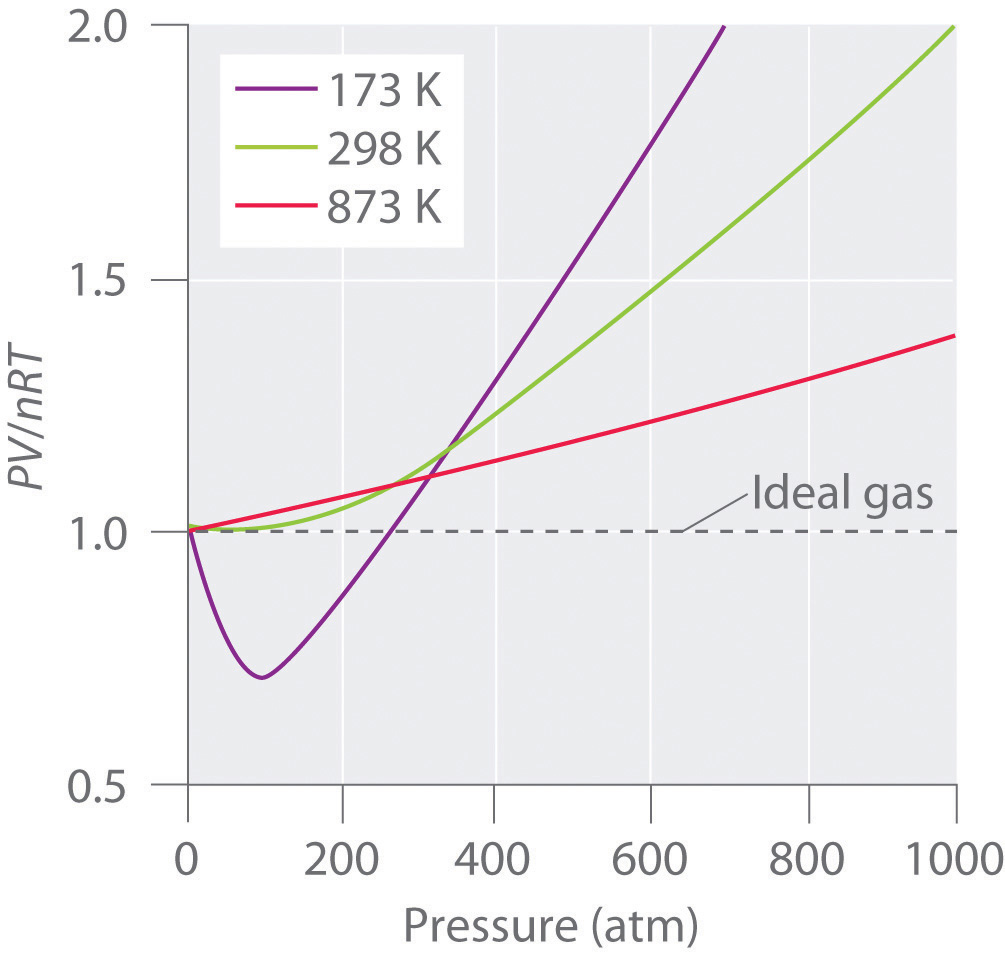
The ratio of volume of real gas v real to the ideal volume of that gas v perfect calculated by ideal gas equation is known as compressibility factor. Real gases have velocity volume and mass. Although the law describes the behavior of an ideal gas the equation is applicable to real gases under many conditions so it is a useful equation to learn to use.
The associated molecules have interactions and space. In contrast real gas molecules have a size and a volume. An ideal gas is different from a real gas in many ways.
This is because an ideal gas is said to be a particle and particles do not have any mass. Real gases are composed of atoms or molecules resulting in their volume. Pv perfect nrt.
When they are cooled to their boiling point they liquefy.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqtkc Gyl1p2tqex6qp Ohddbww3jsg16ffaydfvt3agxnejflg Usqp Cau
Real Gases Vs Ideal Gases Ins Ssrenterprises Co
 Difference Between Ideal Gas Law And Real Gas Law Compare The
Difference Between Ideal Gas Law And Real Gas Law Compare The
 Molecular Formulas And Nomenclature
Molecular Formulas And Nomenclature
 Thermal Properties And Ideal Gases Ideal Gas Law And General Gas
Thermal Properties And Ideal Gases Ideal Gas Law And General Gas
 Behaviour Of Real Gases Deviation From Real Behaviour Videos
Behaviour Of Real Gases Deviation From Real Behaviour Videos
 9 6 Non Ideal Gas Behavior Chemistry
9 6 Non Ideal Gas Behavior Chemistry
 Behaviour Of Real Gases Deviation From Real Behaviour Videos
Behaviour Of Real Gases Deviation From Real Behaviour Videos
 Ideal Gases Vs Real Gases Schoolworkhelper
Ideal Gases Vs Real Gases Schoolworkhelper
 Ideal Gas And Real Gas In Hindi Hindi States Of Matter
Ideal Gas And Real Gas In Hindi Hindi States Of Matter
 Which Gas Is Easier To Compress The Ideal Gas Or A Real Gas
Which Gas Is Easier To Compress The Ideal Gas Or A Real Gas
 Ppt Ch 13 Notes Gas Laws Powerpoint Presentation Free
Ppt Ch 13 Notes Gas Laws Powerpoint Presentation Free
 Could You Help Explain Me This Graph About Ideal Gas And Real
Could You Help Explain Me This Graph About Ideal Gas And Real
/149263439-56a12f093df78cf7726836ed.jpg) Ideal Gas Vs Non Ideal Gas Example Problem
Ideal Gas Vs Non Ideal Gas Example Problem
 Solved Behavior Of Real Vs Ideal Gases A Real Gases Have
Solved Behavior Of Real Vs Ideal Gases A Real Gases Have
 9 6 Non Ideal Gas Behavior Chemistry
9 6 Non Ideal Gas Behavior Chemistry
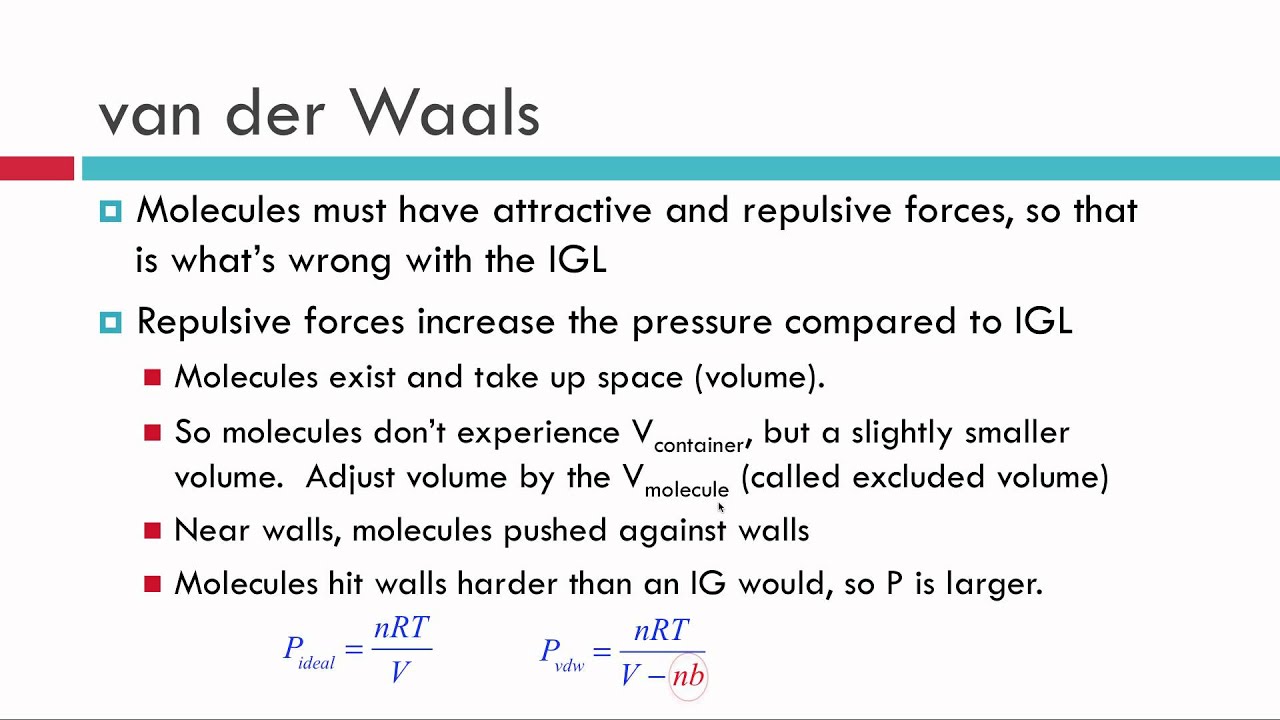 Real Vs Ideal Gases Van Der Waals Explained Youtube
Real Vs Ideal Gases Van Der Waals Explained Youtube
Deviations From Ideal Gas Law Behavior
 Physics Tok Question 2 Eduardo S Tok Blog Year 12
Physics Tok Question 2 Eduardo S Tok Blog Year 12
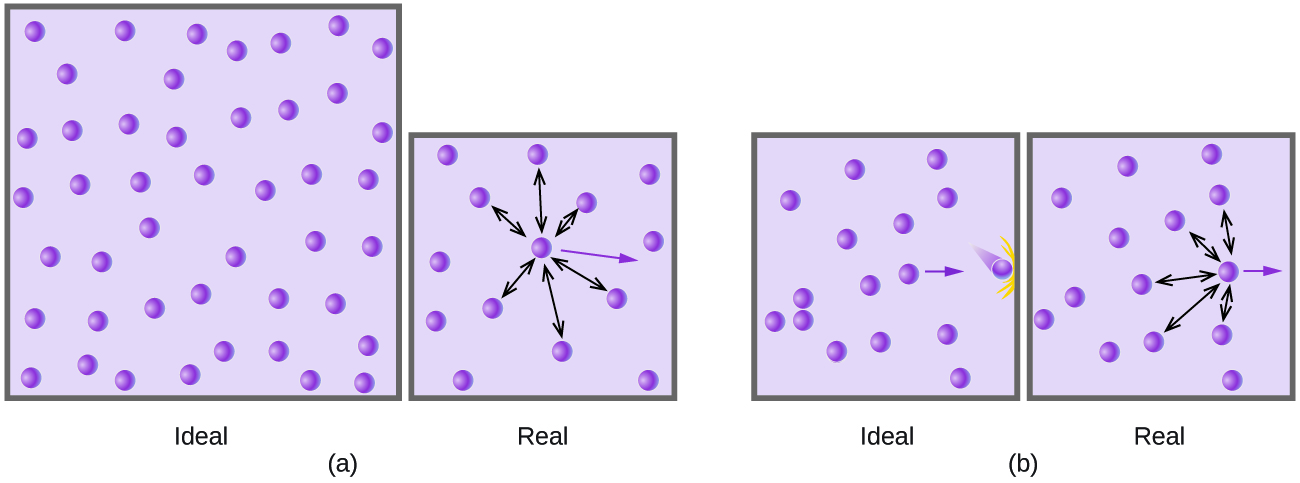 9 6 Non Ideal Gas Behavior Chemistry Libretexts
9 6 Non Ideal Gas Behavior Chemistry Libretexts
 Chapter 10 Gas Laws Objectives Understand The Characteristics Of
Chapter 10 Gas Laws Objectives Understand The Characteristics Of
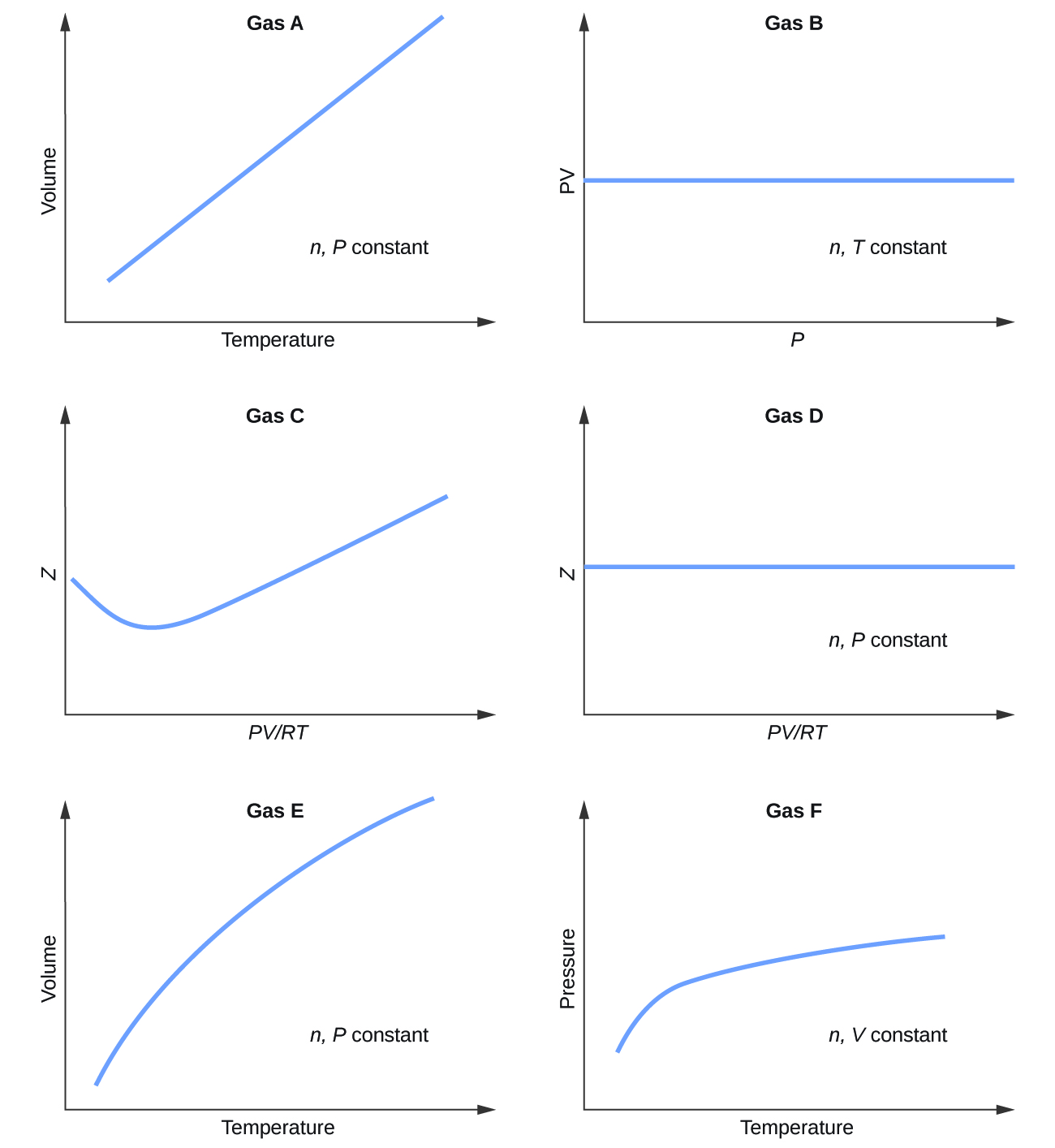 9 6 Non Ideal Gas Behavior Chemistry
9 6 Non Ideal Gas Behavior Chemistry
 Why Doesn T Helium Behave As An Ideal Gas Physics Stack Exchange
Why Doesn T Helium Behave As An Ideal Gas Physics Stack Exchange

Can The Ideal Gas Law Be Applied To Liquids Socratic
Chapter 13 The Behavior Of Gases
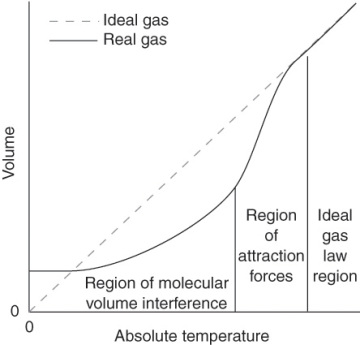 4 5 Real Gases Gas Laws Pressure Volume And Temperature
4 5 Real Gases Gas Laws Pressure Volume And Temperature
 Comparison Of R134a Property Using Ideal Gas And Real Gas Laws
Comparison Of R134a Property Using Ideal Gas And Real Gas Laws







Posting Komentar
Posting Komentar