Pressure And Volume Relationship Equation
Let s use the following models to make sense of the problem. Or boyle s law is a gas law stating that the pressure and volume of a gas have an inverse relationship.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqoint7npybr2 Myq5tea13 Nwnaqinuy67vs Tzjilmylj6omb Usqp Cau
First law of thermodynamics introduction.

Pressure and volume relationship equation. In thermodynamics the relation between pressure and density is expressed through equation of states for. A the graph of p vs. Boyle s law told us that the volume and pressure of an ideal gas had an inversely proportionate relationship.
More on internal energy. Determine pressure and velocity within a cold leg of primary piping and. How to use equation 1 to calculate gas volume or pressure.
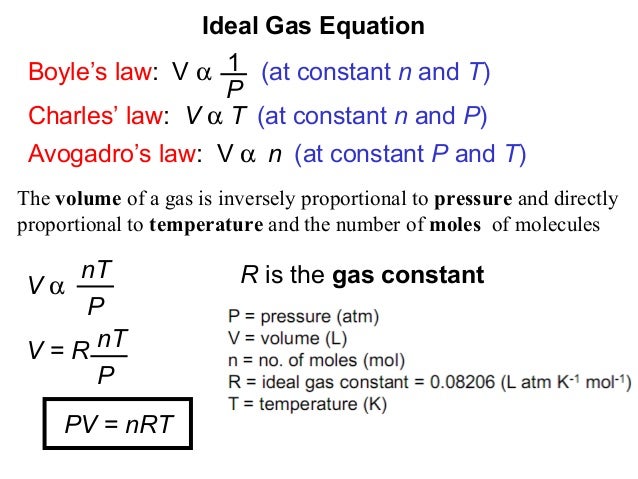
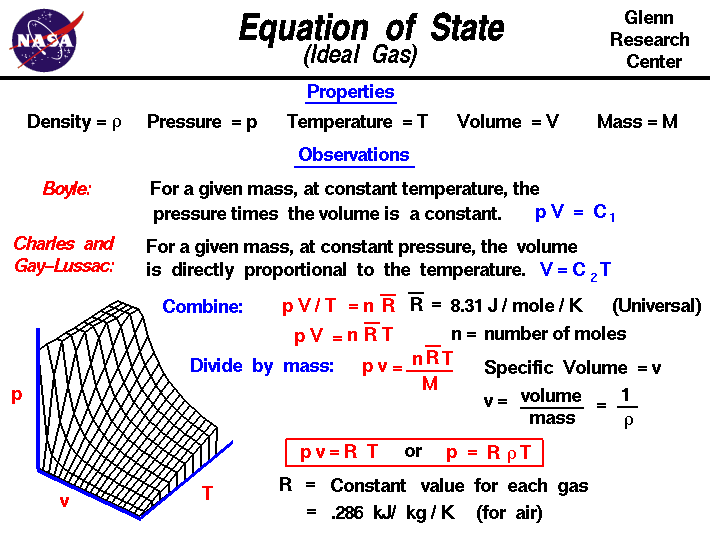
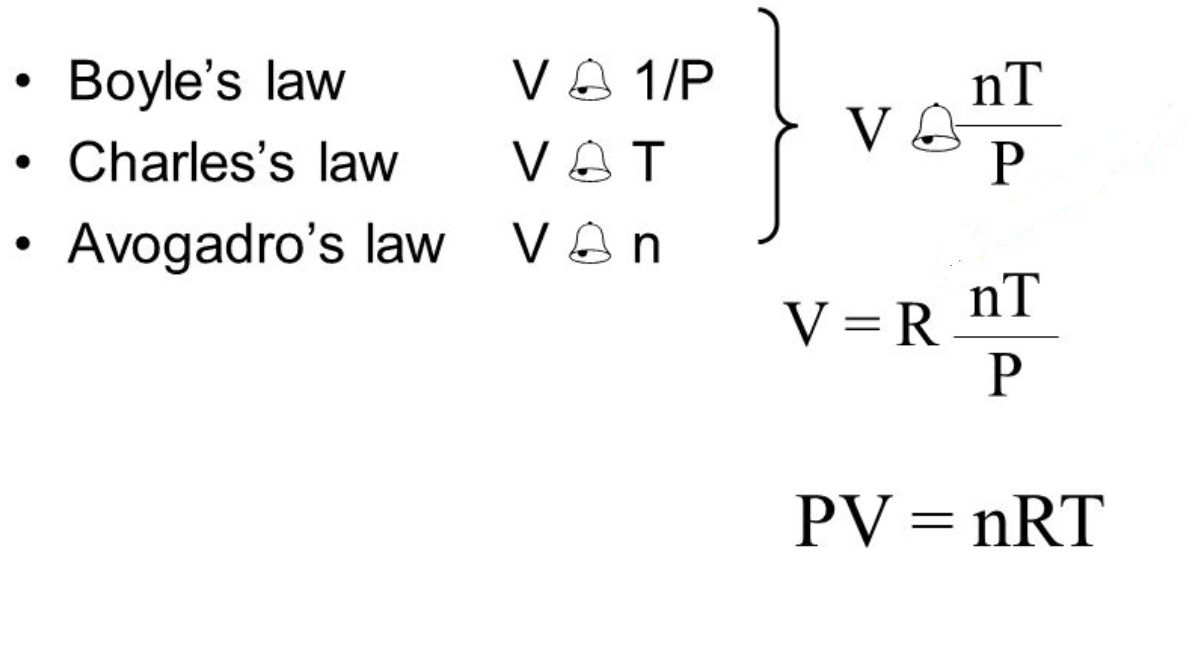
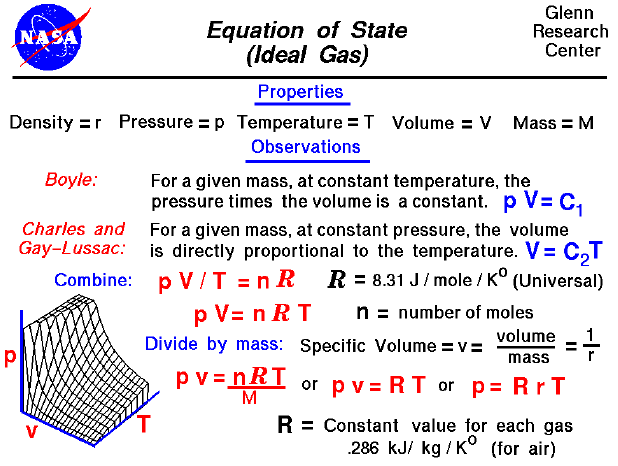
Equation of state ideal gas. Deriving pressure and density equation is very important to understand the concept. The relationship between the volume and pressure of a given amount of gas at constant temperature was first published by the english natural philosopher robert boyle over 300 years ago.
Google classroom facebook twitter. V is a hyperbola whereas b the graph of 1 p vs. Pressure and density equation.
If volume increases then pressure decreases and vice versa when the temperature is held constant. As volume decrease pressure increase. Gay lussac s law amontons law or the pressure law was found by joseph louis gay lussac in 1808.
As one goes up the other goes down. For a fixed mass of an ideal gas kept at a fixed temperature pressure and volume are inversely proportional. The meaning of work in thermodynamics and how to calculate work done by the compression or expansion of a gas.
The relationship between pressure and volume is inversely proportional. As a mathematical equation gay lussac s law is written as either. It states that for a given mass and constant volume of an ideal gas the pressure exerted on the sides of its container is directly proportional to its absolute temperature.
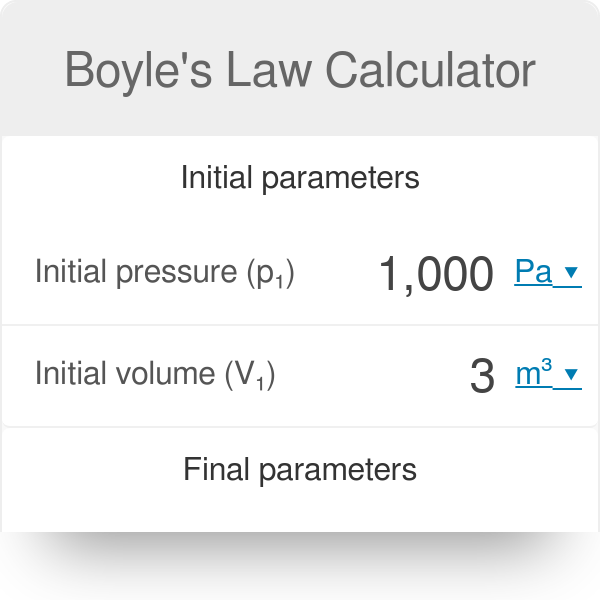
At constant temperature of 35 ºc a sample of gas occupies a volume of 5 0 l and has a pressure of 2 atm. The continuity equation is simply a mathematical expression of the principle of conservation of mass for a control volume that has a single inlet and a single outlet the principle of conservation of mass states that for steady state flow the mass flow rate into the volume must equal the mass flow rate out. Below is the derivation of pressure and density relation for the ideal gas as well as for fluids.
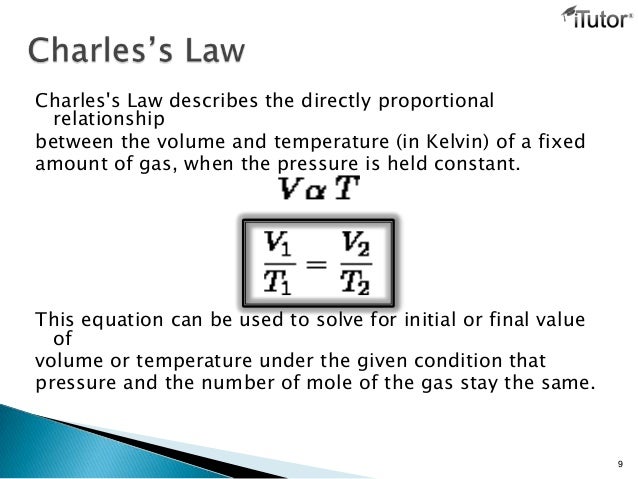
The relationship of a gas with pressure and volume was developed by the scientist robert boyle at around 1660 and is known as boyle s law. As it turns out charles s law tells us that volume tends to sleep around since it is also having a directly proportionate relationship with temperature. Calculating internal energy and work example.
If the volume of the gas decreased to 2 0 l calculate its new pressure. For a fixed mass of gas at a constant temperature the product pressure x volume is a constant.
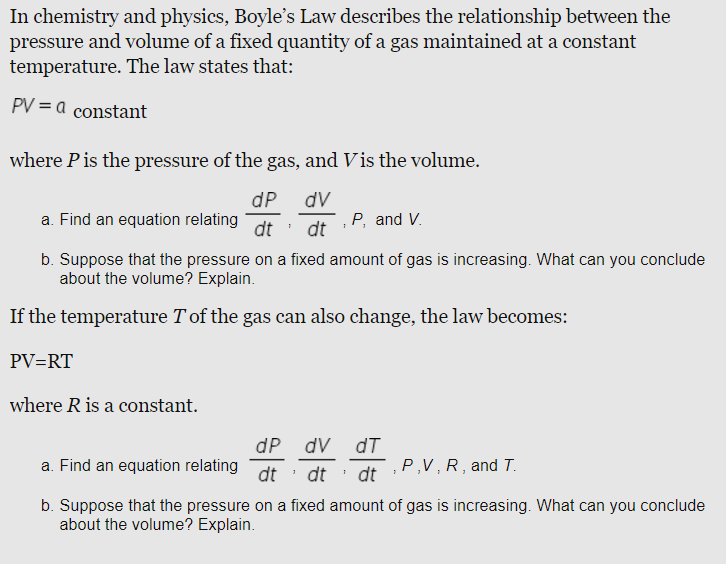 Solved In Chemistry And Physics Boyle S Law Describes Th
Solved In Chemistry And Physics Boyle S Law Describes Th
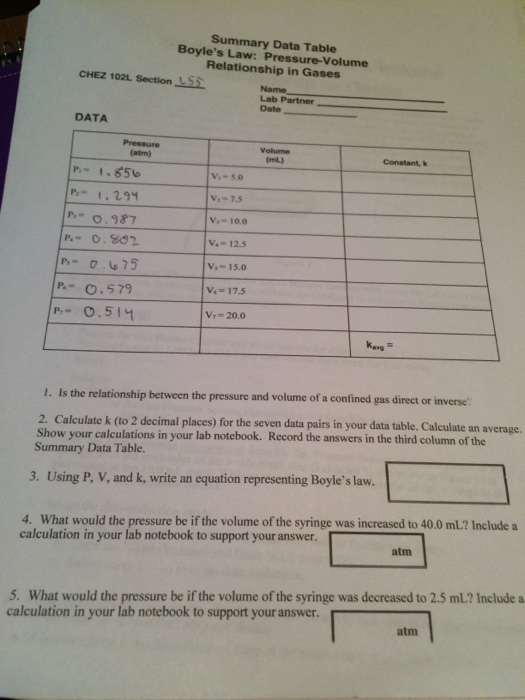
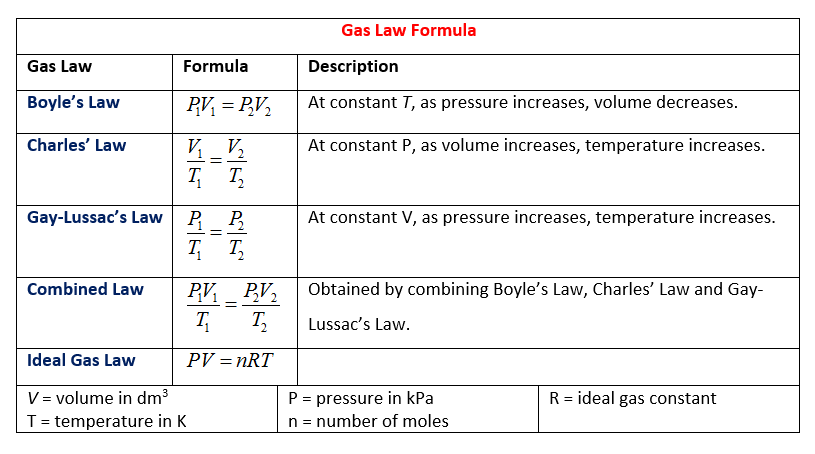 Gas Laws Solutions Examples Worksheets Videos Games Activities
Gas Laws Solutions Examples Worksheets Videos Games Activities
 Title Lesson 9 Relationship Between Volume Temperature And
Title Lesson 9 Relationship Between Volume Temperature And
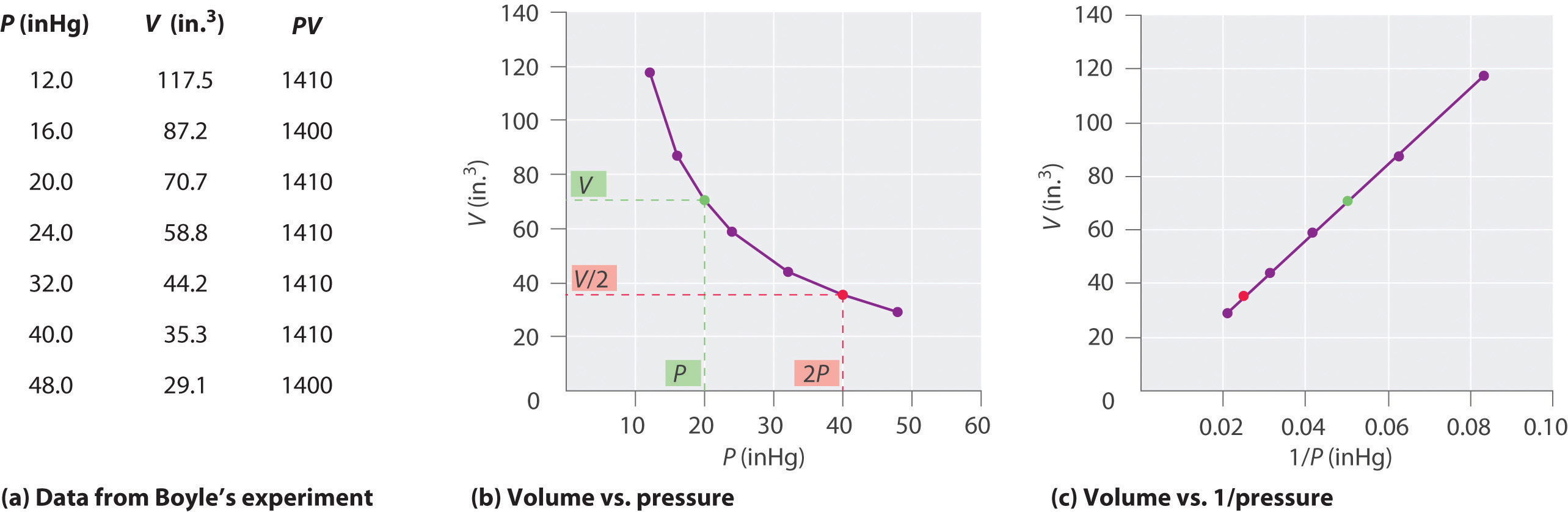 6 3 Relationships Among Pressure Temperature Volume And Amount
6 3 Relationships Among Pressure Temperature Volume And Amount
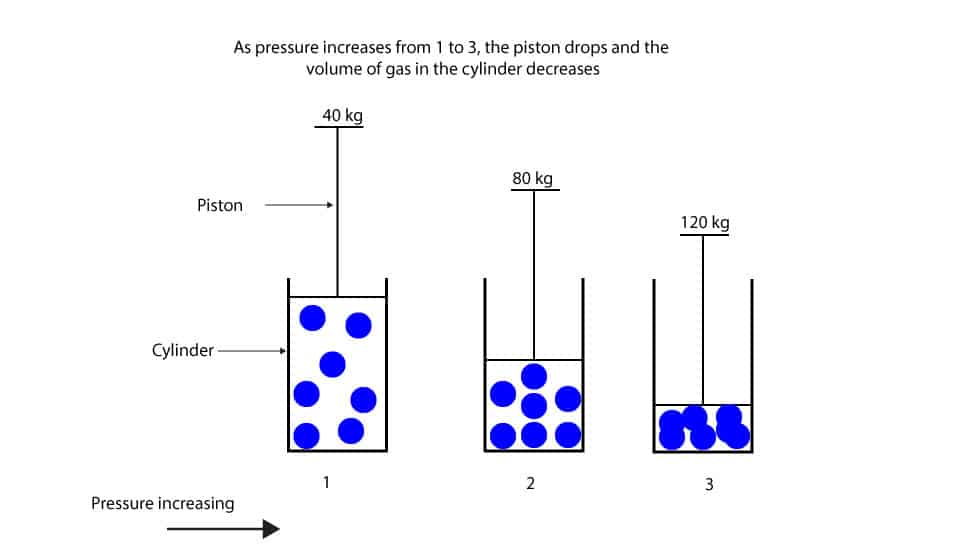 What S The Relationship Between Pressure And Volume Of Gas
What S The Relationship Between Pressure And Volume Of Gas
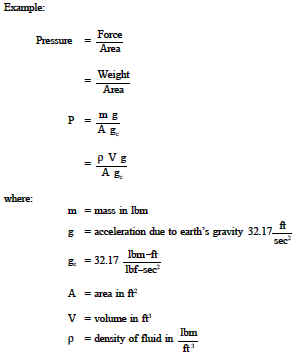 Relationship Between Depth And Pressure Review And Equations
Relationship Between Depth And Pressure Review And Equations
 Solved The Ideal Gas Equation Has Been Widely Used To Rel
Solved The Ideal Gas Equation Has Been Widely Used To Rel
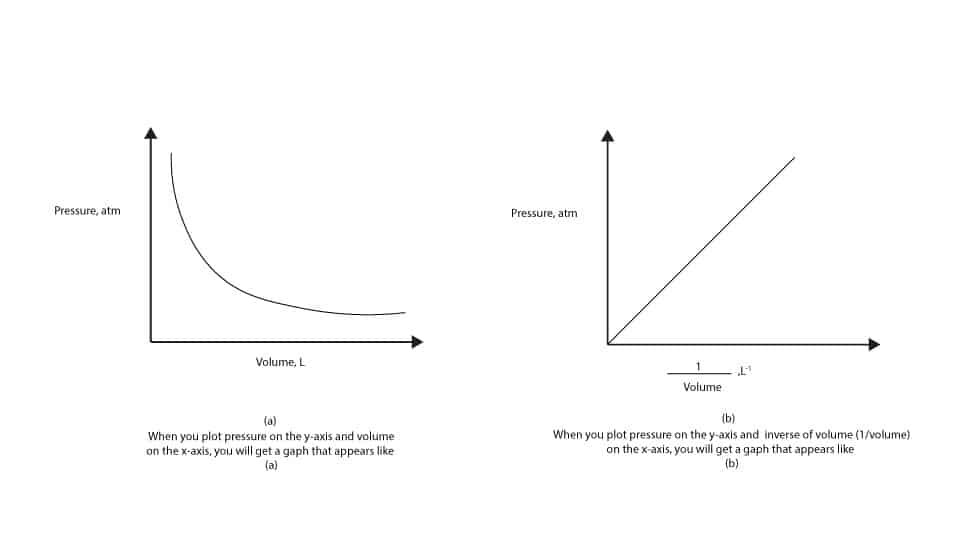 What S The Relationship Between Pressure And Volume Of Gas
What S The Relationship Between Pressure And Volume Of Gas
What Is The Relationship Between Pressure Volume And Temperature
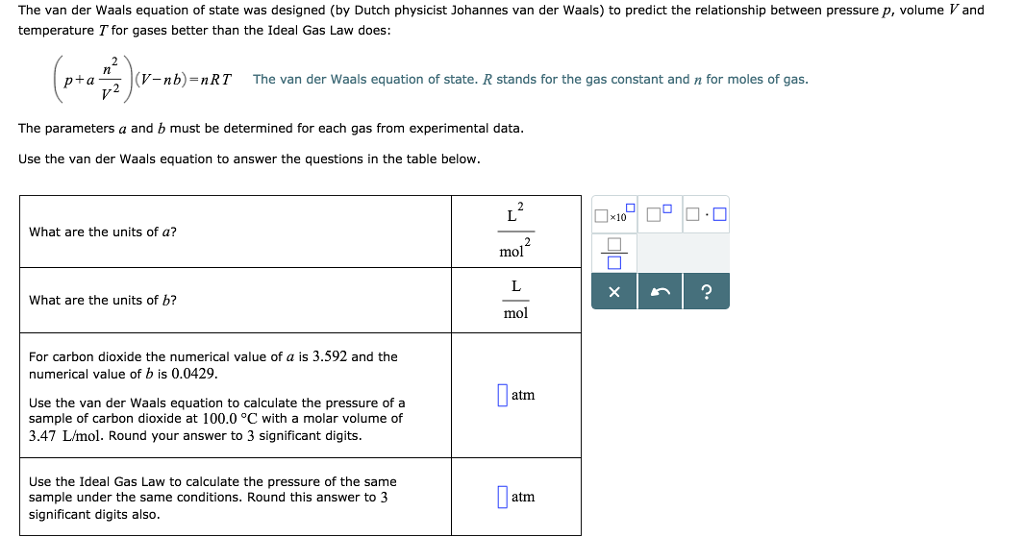 Solved The Van Der Waals Equation Of State Was Designed
Solved The Van Der Waals Equation Of State Was Designed
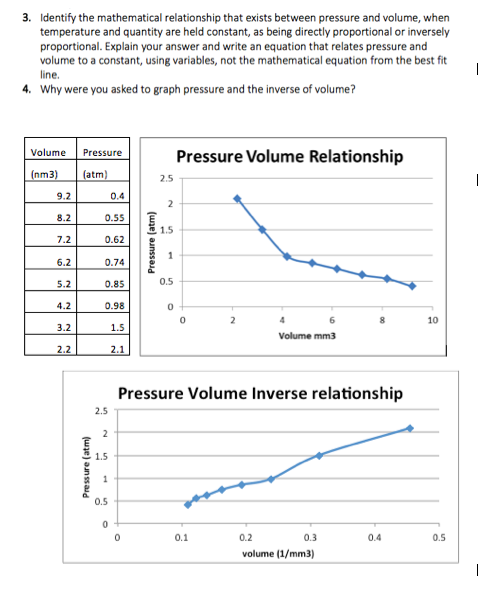 Solved 3 Identify The Mathematical Relationship That Exi
Solved 3 Identify The Mathematical Relationship That Exi
 Pressure Volume And Temperature Relationships Chemistry
Pressure Volume And Temperature Relationships Chemistry
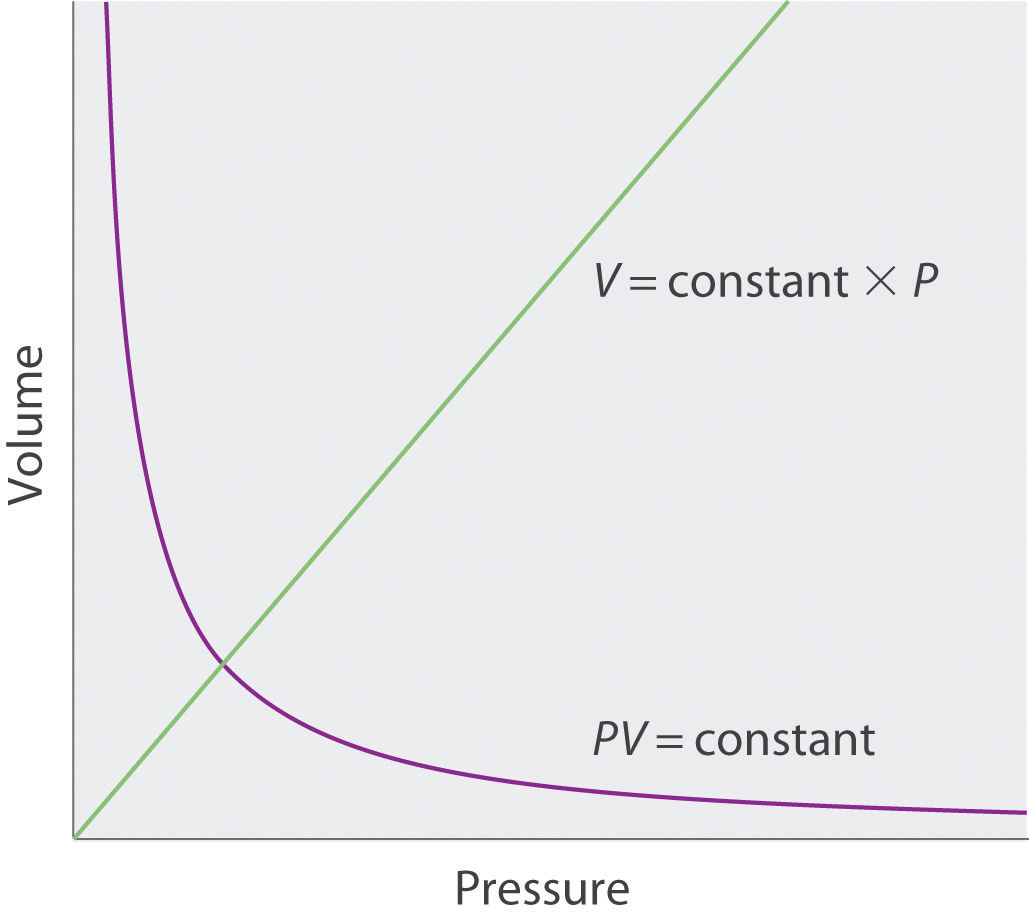 Relationships Among Pressure Temperature Volume And Amount
Relationships Among Pressure Temperature Volume And Amount
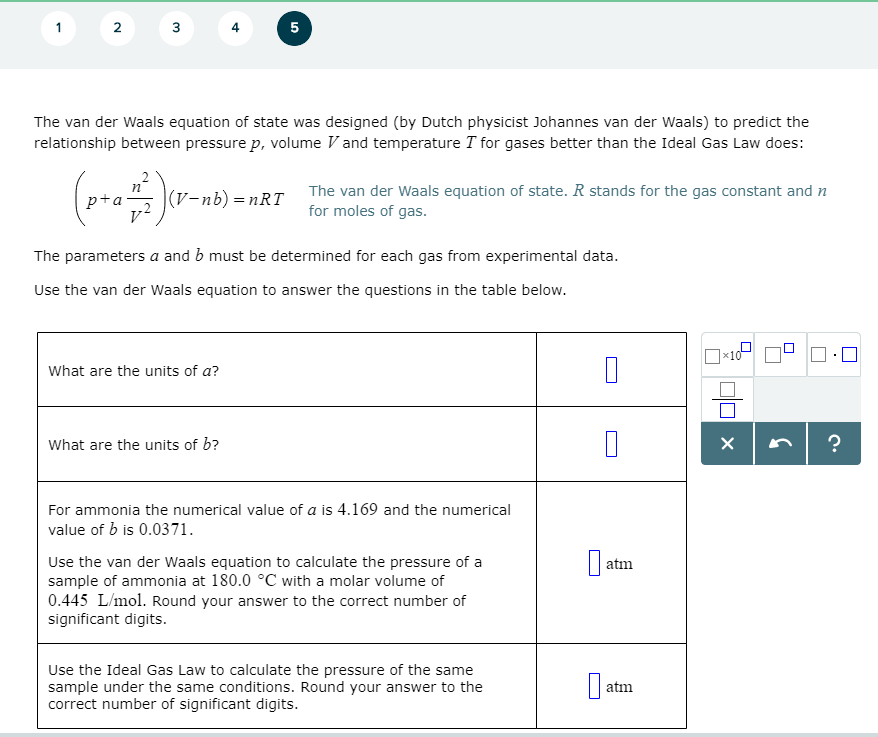 Solved 4 5 The Van Der Waals Equation Of State Was Design
Solved 4 5 The Van Der Waals Equation Of State Was Design
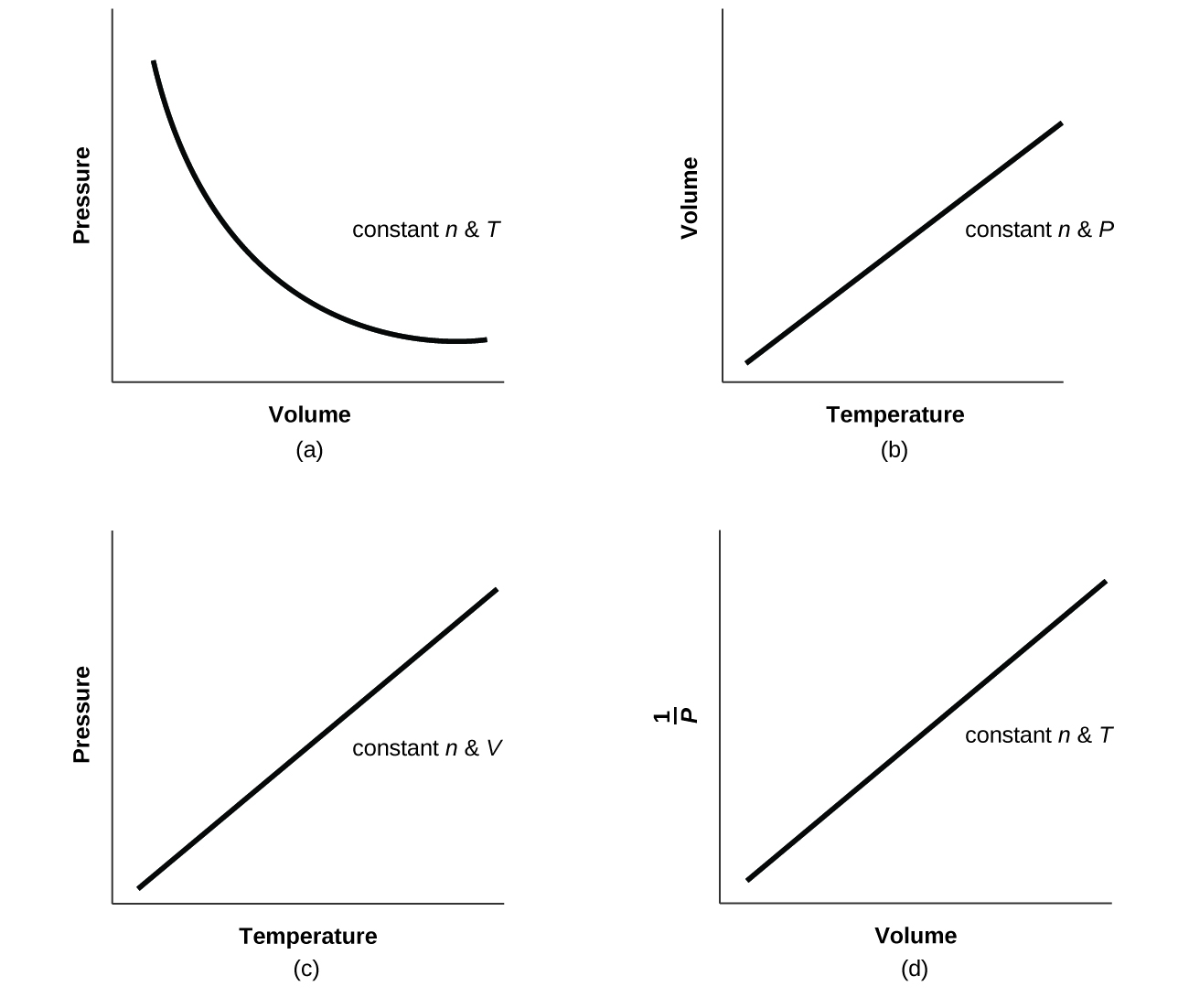 9 2 Relating Pressure Volume Amount And Temperature The Ideal
9 2 Relating Pressure Volume Amount And Temperature The Ideal
 1 Equations Of State The Relationship Among The State Variables
1 Equations Of State The Relationship Among The State Variables
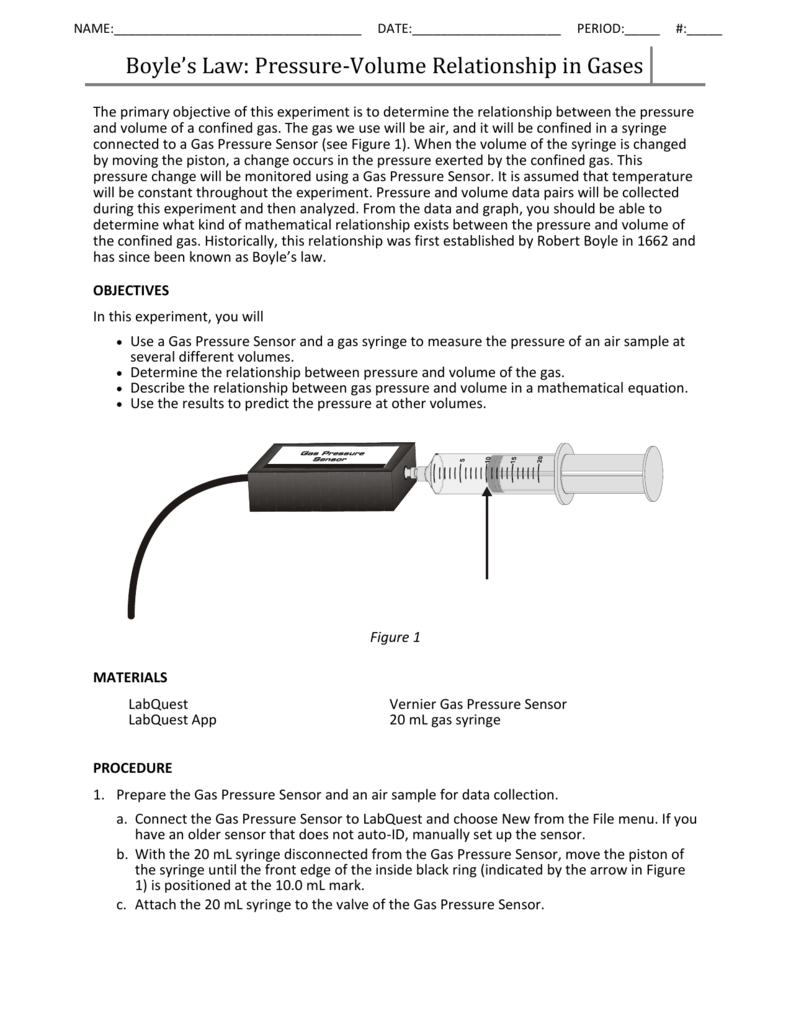 Boyle S Law Pressure Volume Relationship In Gases
Boyle S Law Pressure Volume Relationship In Gases
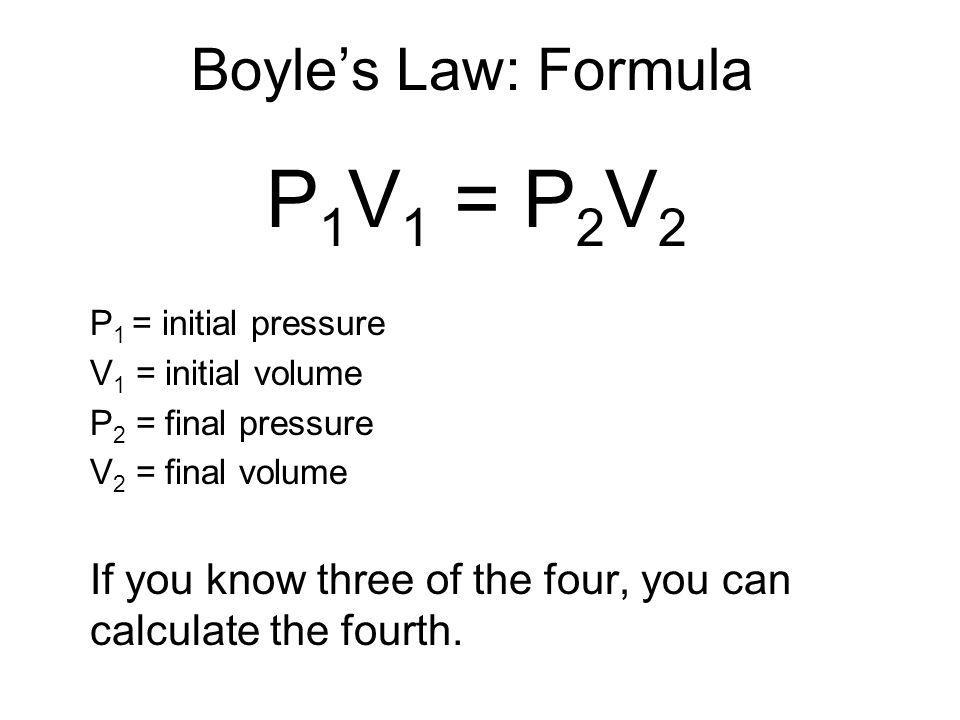 If 2 L Of A Gas At Room Temperature Exerts A Pressure Of 72 Kpa On
If 2 L Of A Gas At Room Temperature Exerts A Pressure Of 72 Kpa On
 Boyle S Law Pressure Volume Relationship In Gases
Boyle S Law Pressure Volume Relationship In Gases
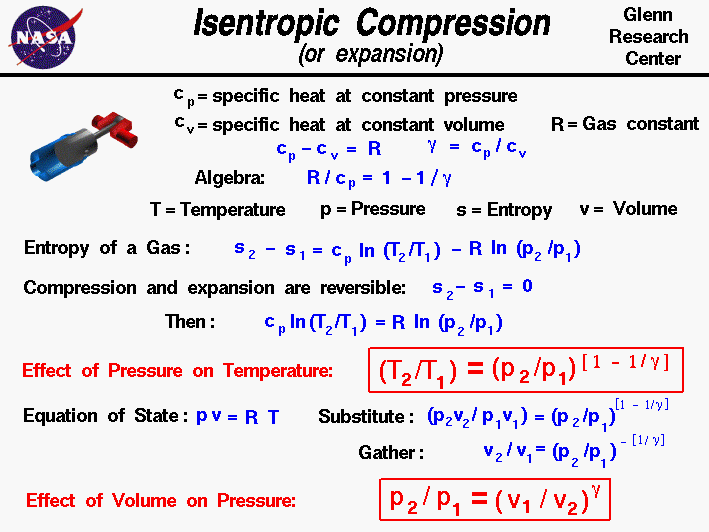 Isentropic Compression Or Expansion
Isentropic Compression Or Expansion
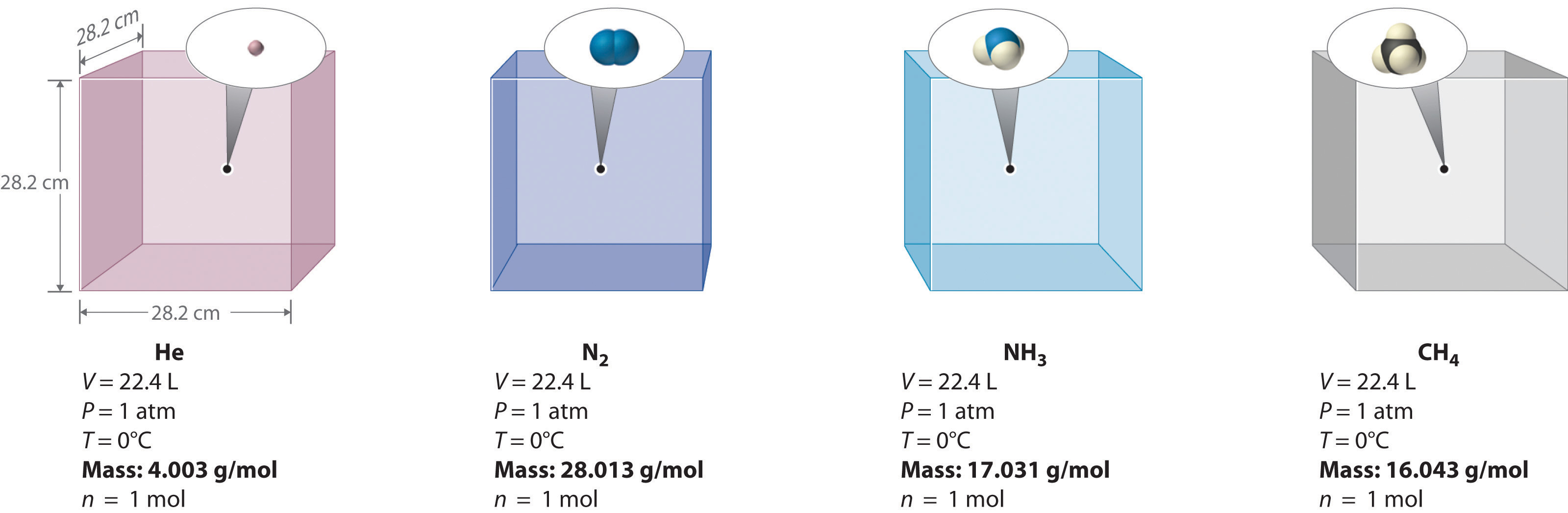 6 3 Relationships Among Pressure Temperature Volume And Amount
6 3 Relationships Among Pressure Temperature Volume And Amount
 Pressure Volume And Temperature Relationships Chemistry
Pressure Volume And Temperature Relationships Chemistry
 Boyle S Law Pressure Volume Relationship In Gases Vernier
Boyle S Law Pressure Volume Relationship In Gases Vernier
 2 2 2 Using The Isobaric Relationship Question 2 Chegg Com
2 2 2 Using The Isobaric Relationship Question 2 Chegg Com
Pressure And Volume Relationship Of A Gas Boyle S Law Pass My
 Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsmuuavcsj6ln2isyzghu7e7i8wwyneooet3g Usqp Cau
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsmuuavcsj6ln2isyzghu7e7i8wwyneooet3g Usqp Cau
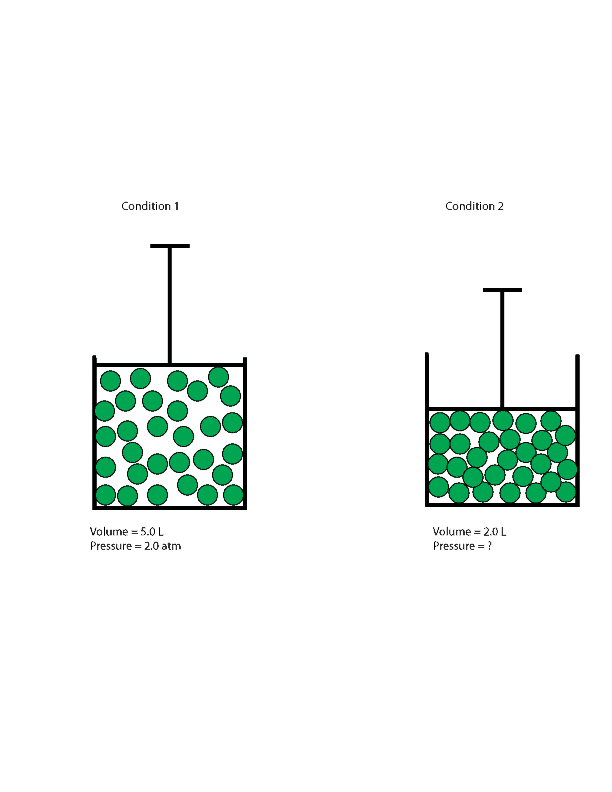 What S The Relationship Between Pressure And Volume Of Gas
What S The Relationship Between Pressure And Volume Of Gas



Posting Komentar
Posting Komentar