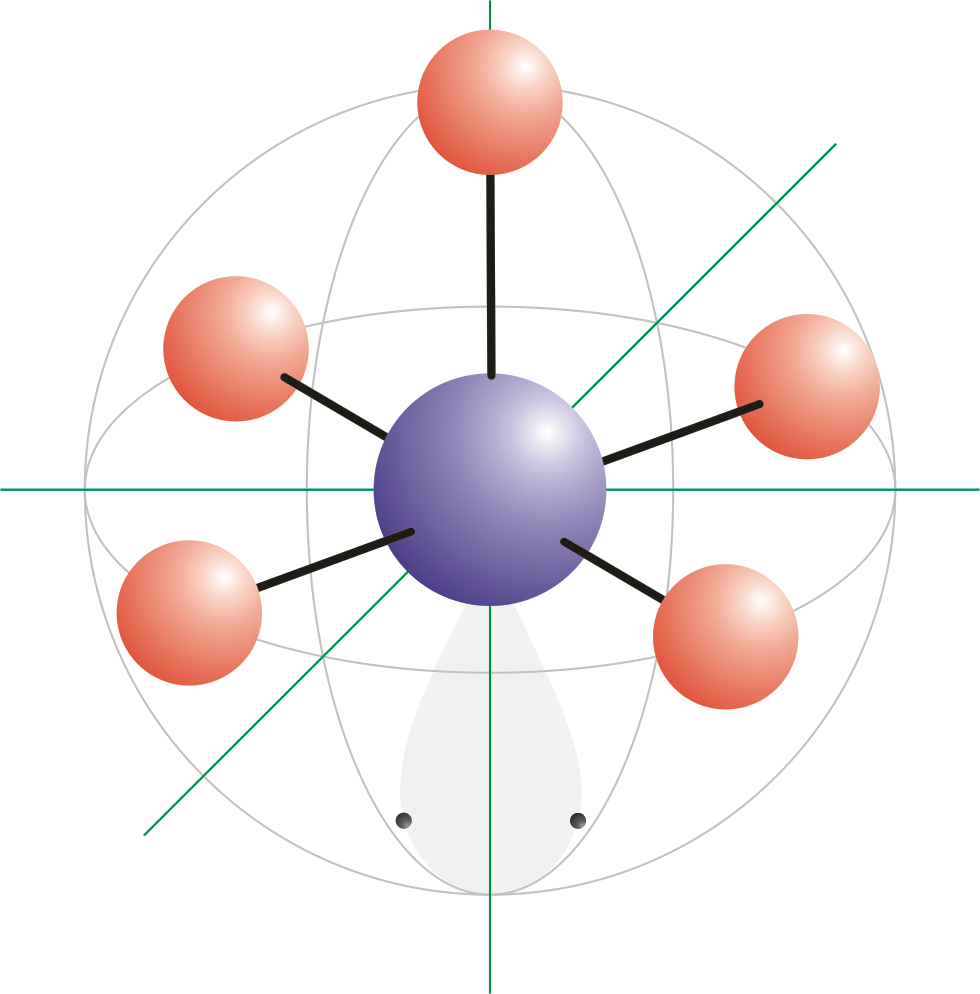
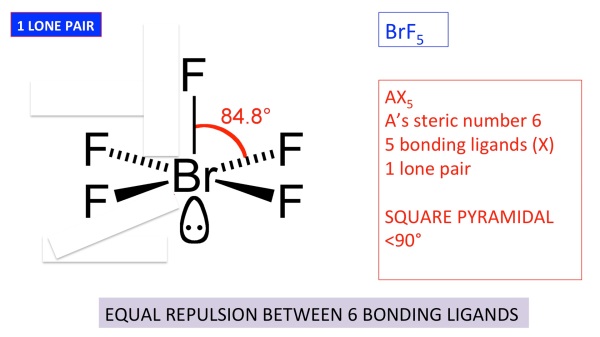
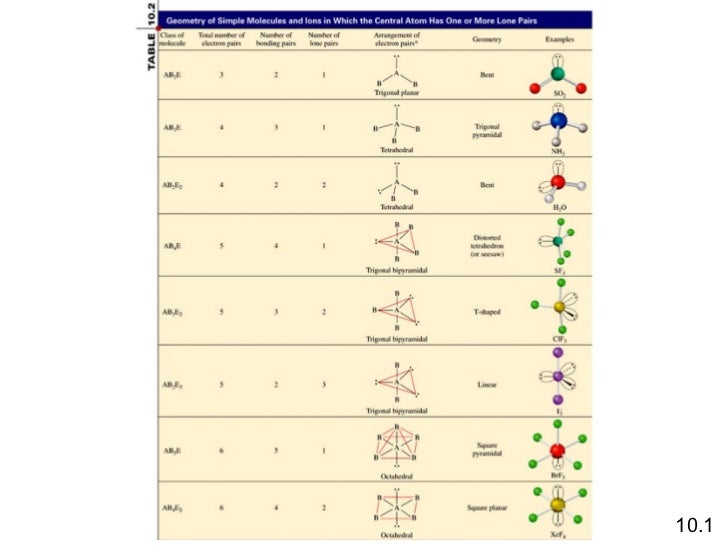
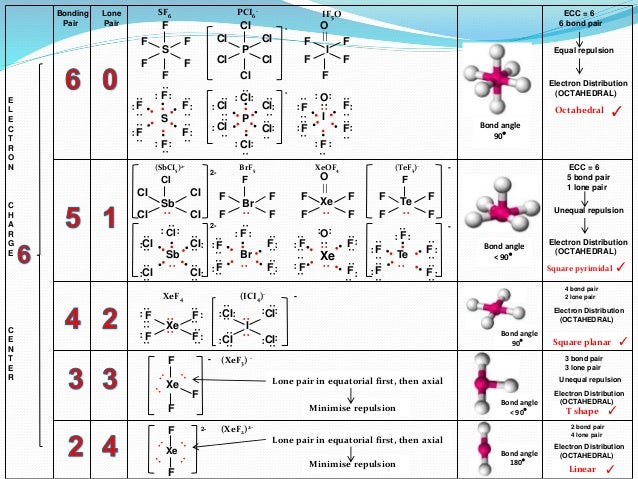
Square Pyramidal Bond Angle
The shape of the orbitals is octahedral. This molecule is made up of 6 equally spaced sp 3 d 2 hybrid orbitals arranged at 90 o angles.
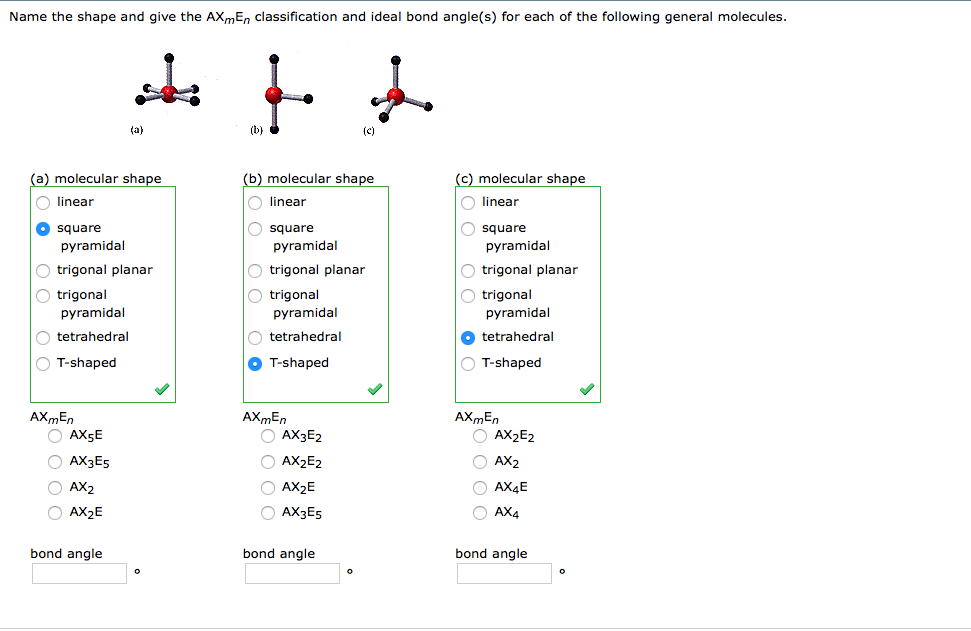 Solved Name The Shape And Give The Ax Me N Classification
Solved Name The Shape And Give The Ax Me N Classification
For example sulfur hexafluoride sf 6 is an octahedral molecule.

Square pyramidal bond angle. One orbital contains a lone pair of electrons so the remaining five atoms connected to the central atom gives the molecule a square pyramidal shape. I sponsored by blissy. The molecular shape is square pyramidal because it has five ligands and one lone pair and the bond angle are 90 lt 120.
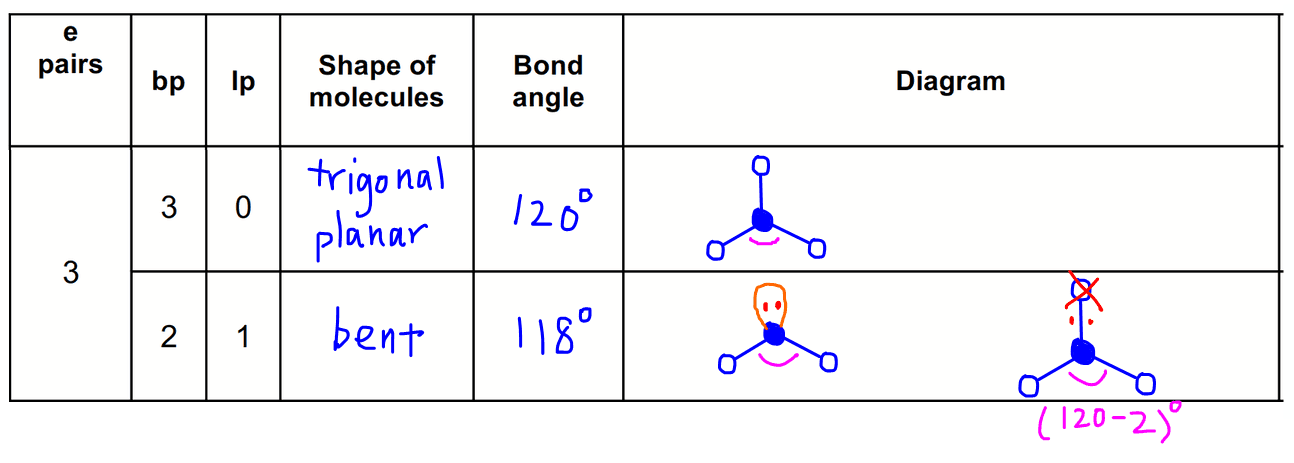
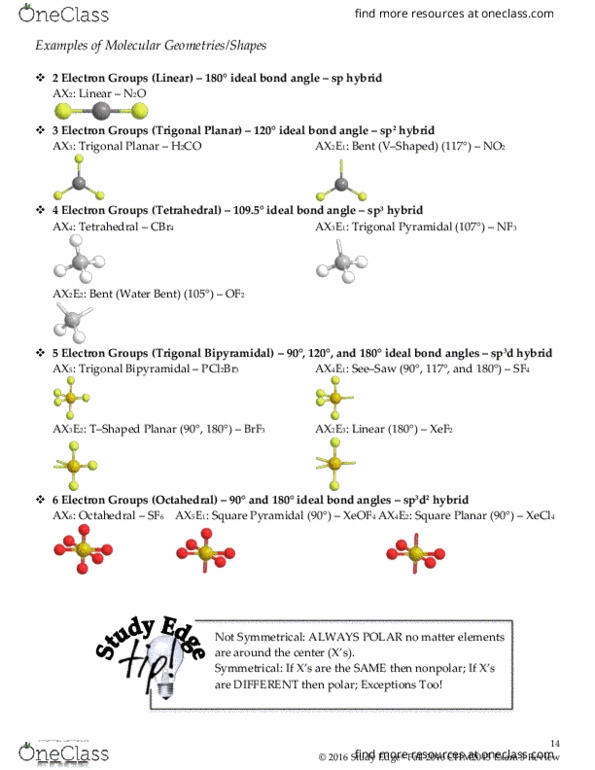
We can draw the lewis structure on a sheet of paper. For trigonal pyramidal geometry the bond angle is slightly less than 109 5 degrees around 107 degrees. Similarly in sp3d2 it forms square bipyramidal axial is at an angle of 180 degree bond angle between equitorial is 90 degree and between axial and equitorial bond angle is 90 degree.
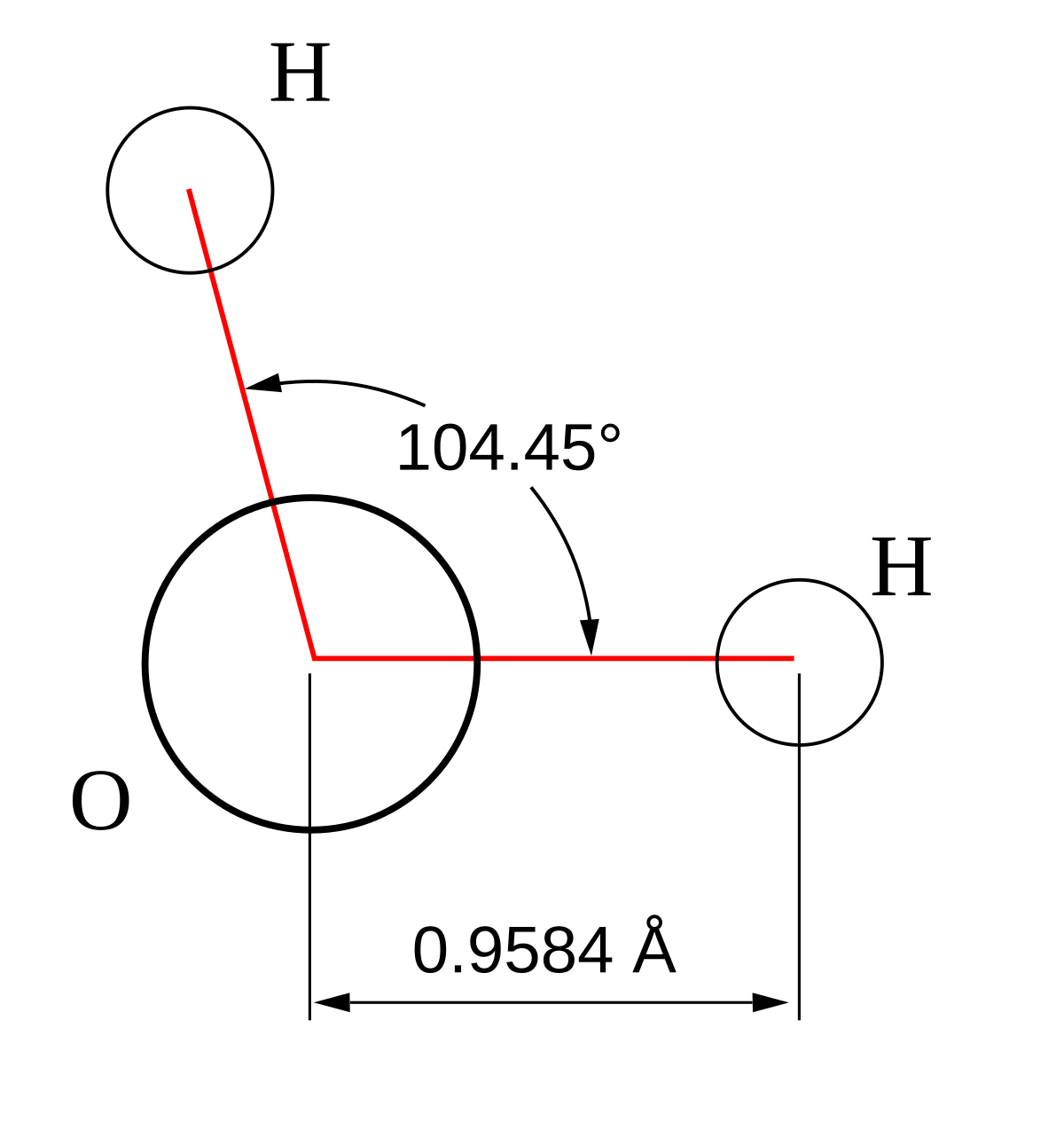
In molecular geometry square pyramidal geometry describes the shape of certain compounds with the formula ml 5 where l is a ligand if the ligand atoms were connected the resulting shape would be that of a pyramid with a square base. A trigonal pyramidal molecule has a pyramid like shape with a triangular base. Water molecule the bond angle for water is 104 5.
Lets consider the lewis structure for ccl 4. 410 və sep ər 2 is a model used in chemistry to predict the geometry of individual. Also it has no dipole moment and the electron group geometry is.
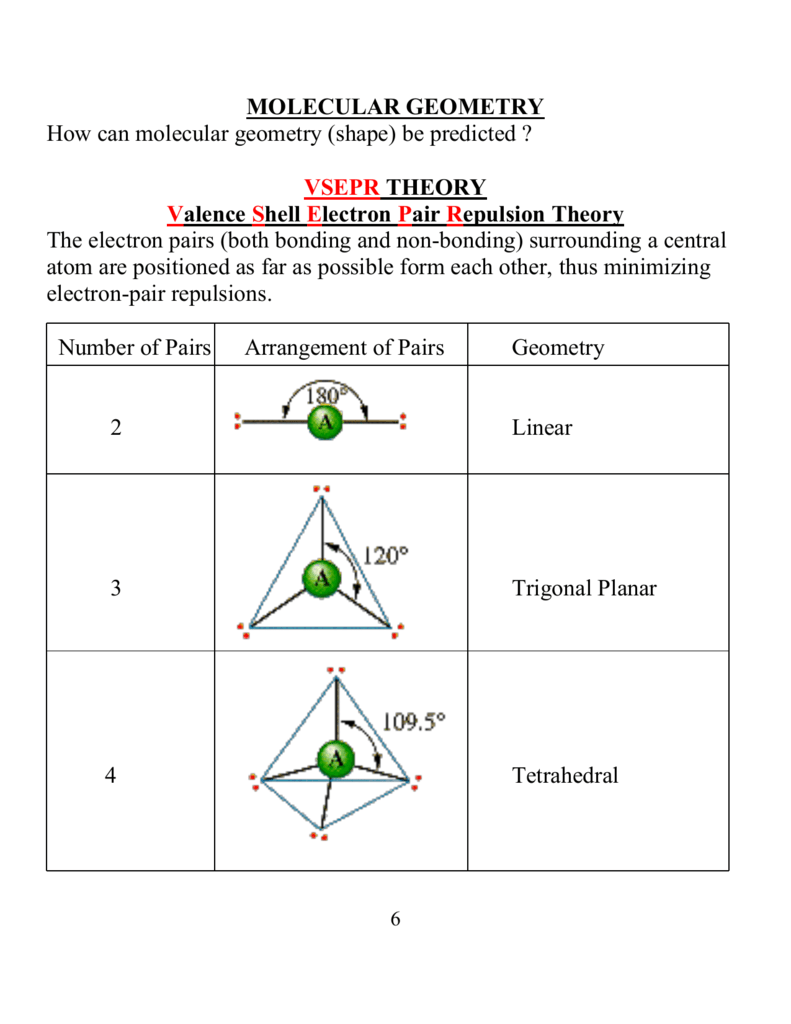
Valence shell electron pair repulsion theory or vsepr theory ˈ v ɛ s p ər v ə ˈ s ɛ p ər vesp ər 1. Shows location of unpaired electrons bonded atoms and bond angles. October 08 2017 molecular shapes and bond angles nh3 bond angle pf5 molecular geometry bcl3 bond angle bonding angles methane bond angle molecular shapes worksheet cf2o bond angle cf4 bond angle electron geometry bond angles vsepr theory bond angles tetrahedral bond angles molecule bond angles sf2 bond angle water bond angle how to find bond angle how to determine bond angle.
For bent molecular geometry when the electron pair geometry is tetrahedral the bond angle is around 105 degrees. The bond angle is 90 degrees. The point group symmetry involved is of type c 4v the geometry is common for certain main group compounds that have a stereochemically active lone pair as.
Ax 5 e notes.
 Chemistry Glossary Search Results For Square Pyramidal Molecular
Chemistry Glossary Search Results For Square Pyramidal Molecular
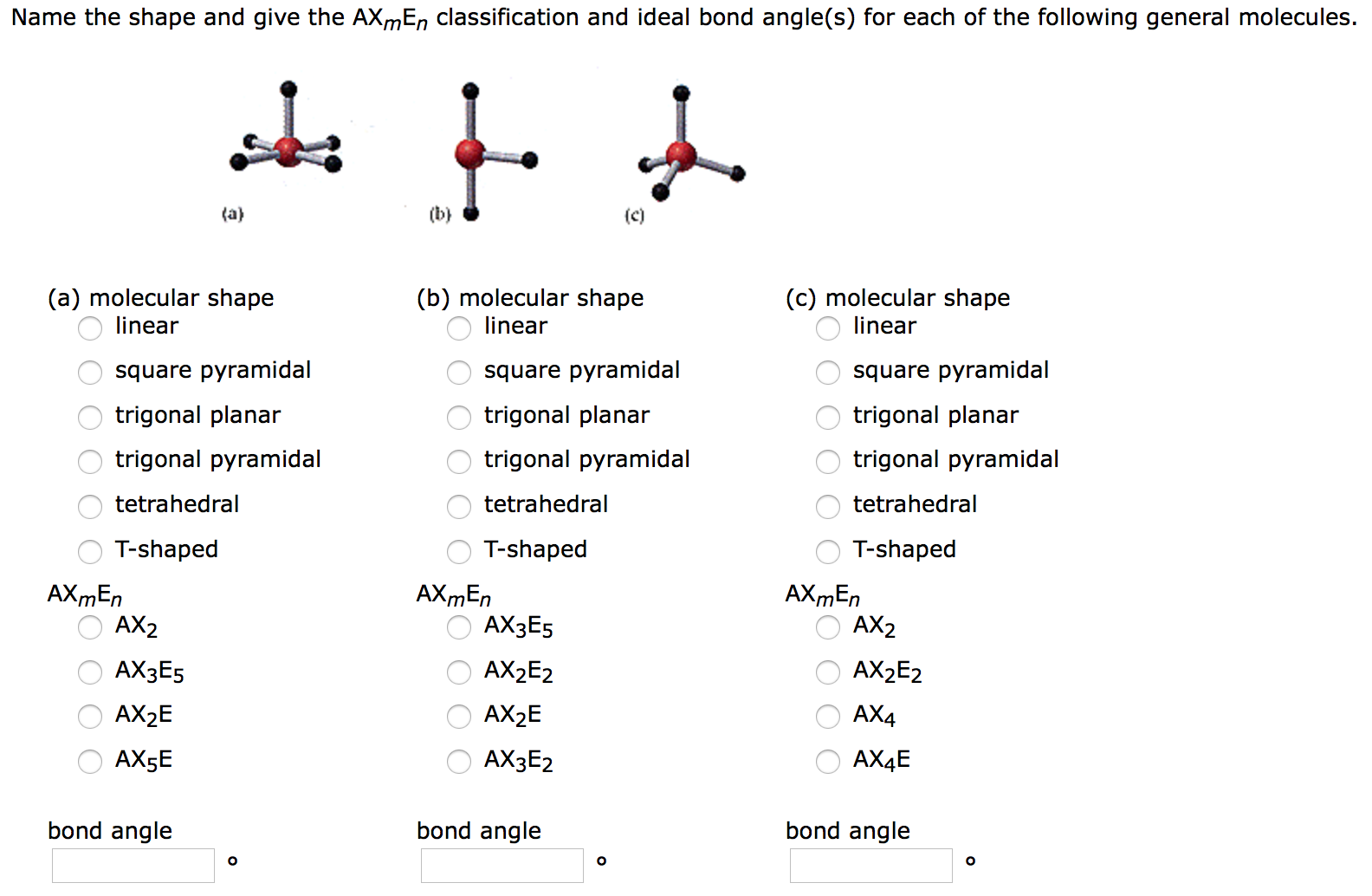 Solved Name The Shape And Give The Axmen Classification A
Solved Name The Shape And Give The Axmen Classification A
 A Model For Clf5 Is Shown In The Chem3d Window Clf5 Has Square
A Model For Clf5 Is Shown In The Chem3d Window Clf5 Has Square
 Hybridization Molecular Geometry And Bond Angles Without With
Hybridization Molecular Geometry And Bond Angles Without With
Chapter 7 Covalent Bonds And Molecular Structure
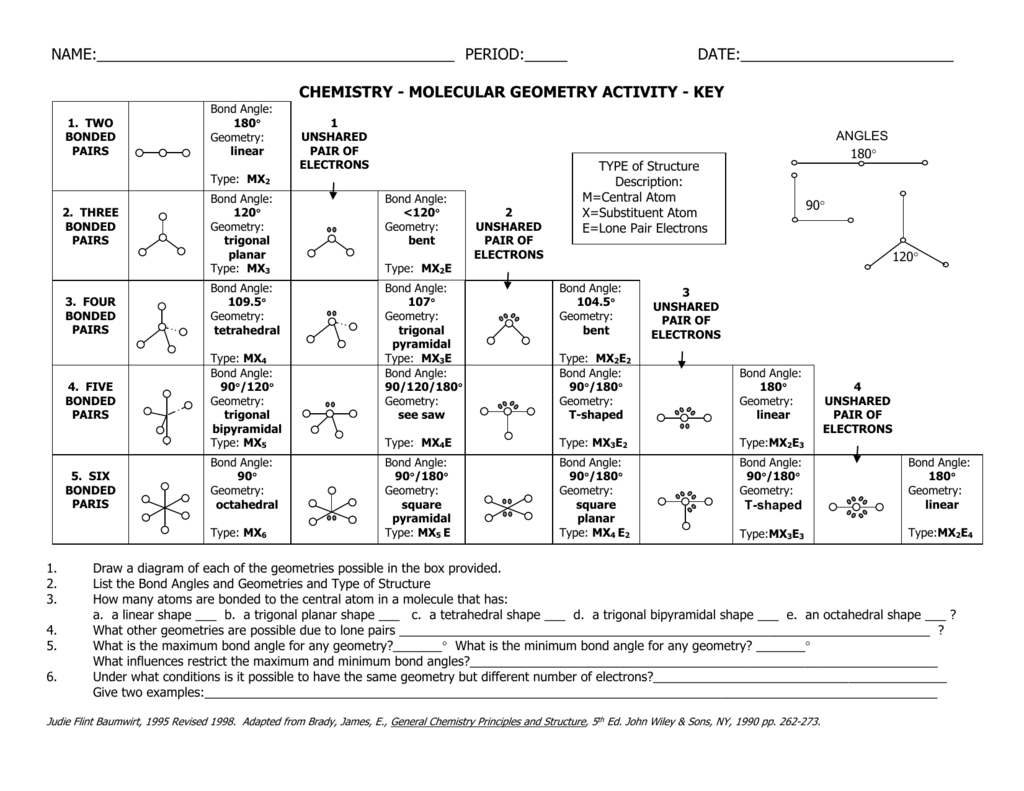 Chemistry Molecular Geometry Activity
Chemistry Molecular Geometry Activity
 Shapes Of Simple Molecule And Ions Secondary Science 4 All
Shapes Of Simple Molecule And Ions Secondary Science 4 All
 The Structure Of Tef 5 Isdraw A Complete Clutch Prep
The Structure Of Tef 5 Isdraw A Complete Clutch Prep
 Chemistry Glossary Search Results For Square Pyramidal Molecular
Chemistry Glossary Search Results For Square Pyramidal Molecular
 Valence Shell Electron Pair Repulsion Theory
Valence Shell Electron Pair Repulsion Theory
Chem College Molecular Shapes And Bond Angle Continued
 And Is It Polar Or Non Polar Draw The Lewis Structure Of Aso2
And Is It Polar Or Non Polar Draw The Lewis Structure Of Aso2
Molecules With Expanded Valence Shells
 Molecular Geometry Boundless Chemistry
Molecular Geometry Boundless Chemistry
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctzax9mrjbmrzffszehvgthiihsqmon1vw9wsuuequgeyuyppm Usqp Cau
 Shapes Of Molecules David Read Key Aims Revise A Level Vsepr
Shapes Of Molecules David Read Key Aims Revise A Level Vsepr
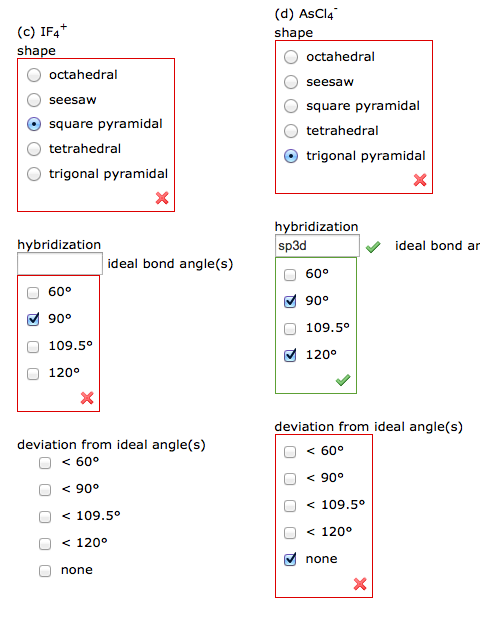 Solved If4 Shape Octahedral Seesaw Square Pyramidal Tetr
Solved If4 Shape Octahedral Seesaw Square Pyramidal Tetr
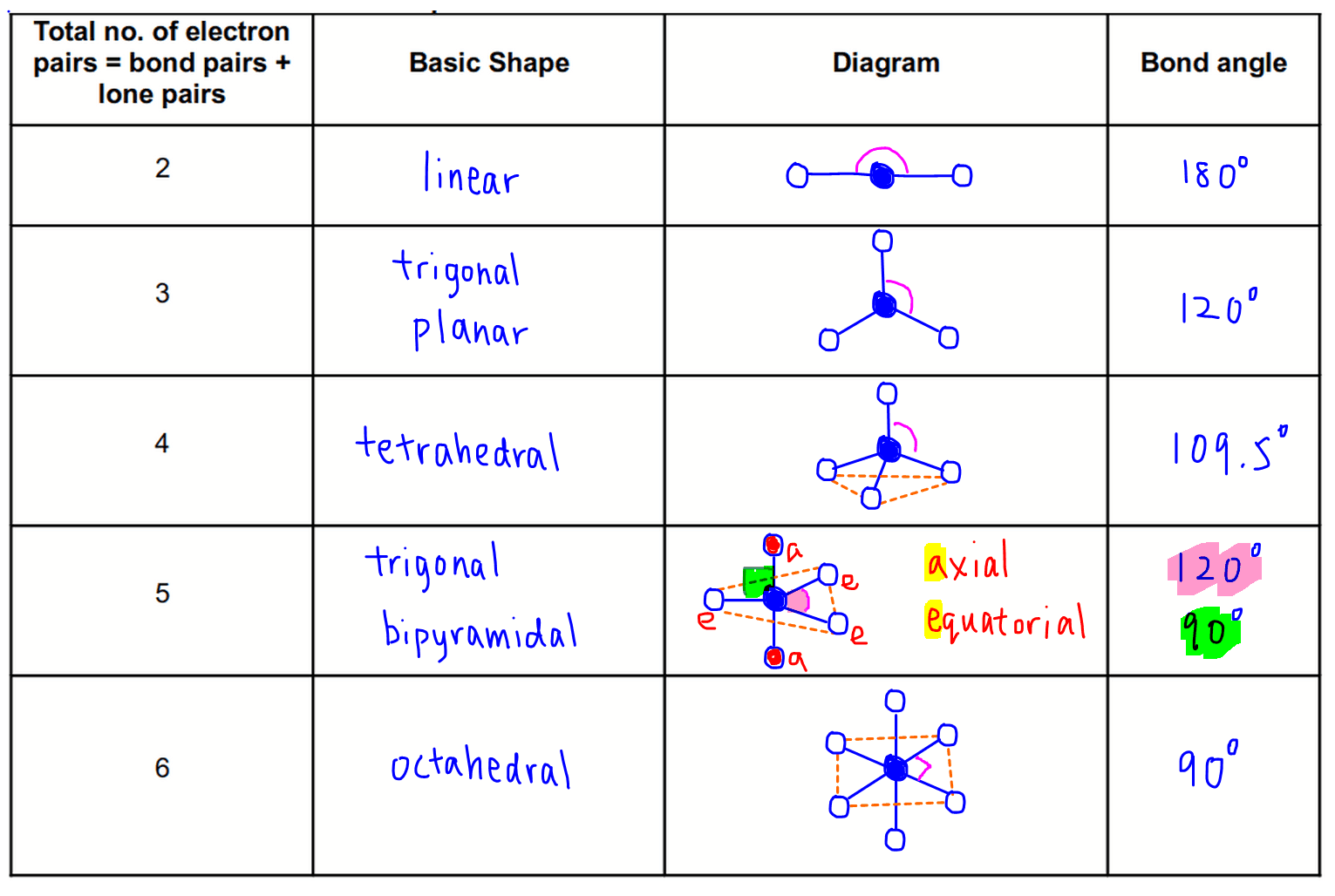 Valence Shell Electron Pair Repulsion Theory
Valence Shell Electron Pair Repulsion Theory
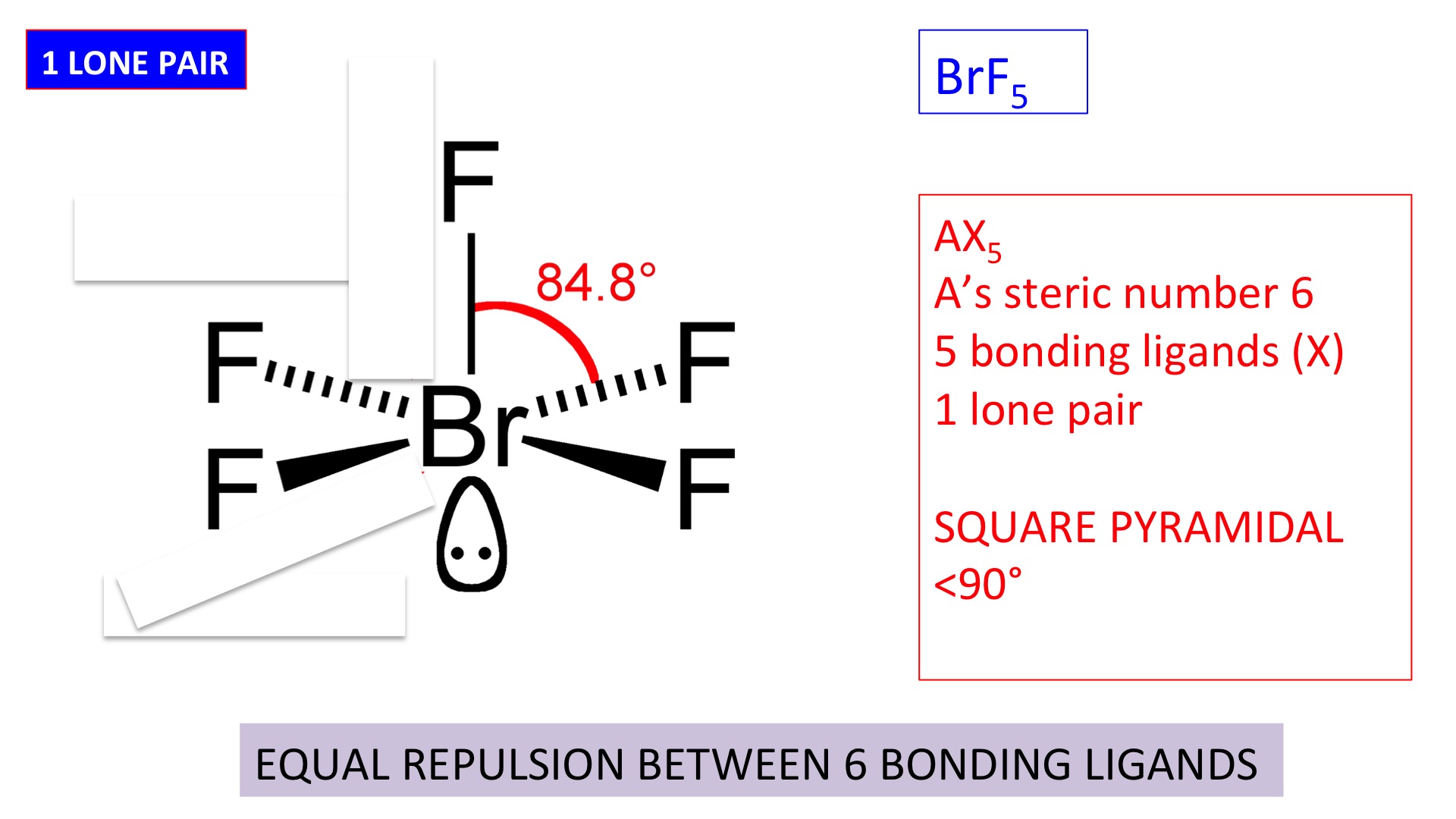 Shapes Of Simple Molecule And Ions Secondary Science 4 All
Shapes Of Simple Molecule And Ions Secondary Science 4 All
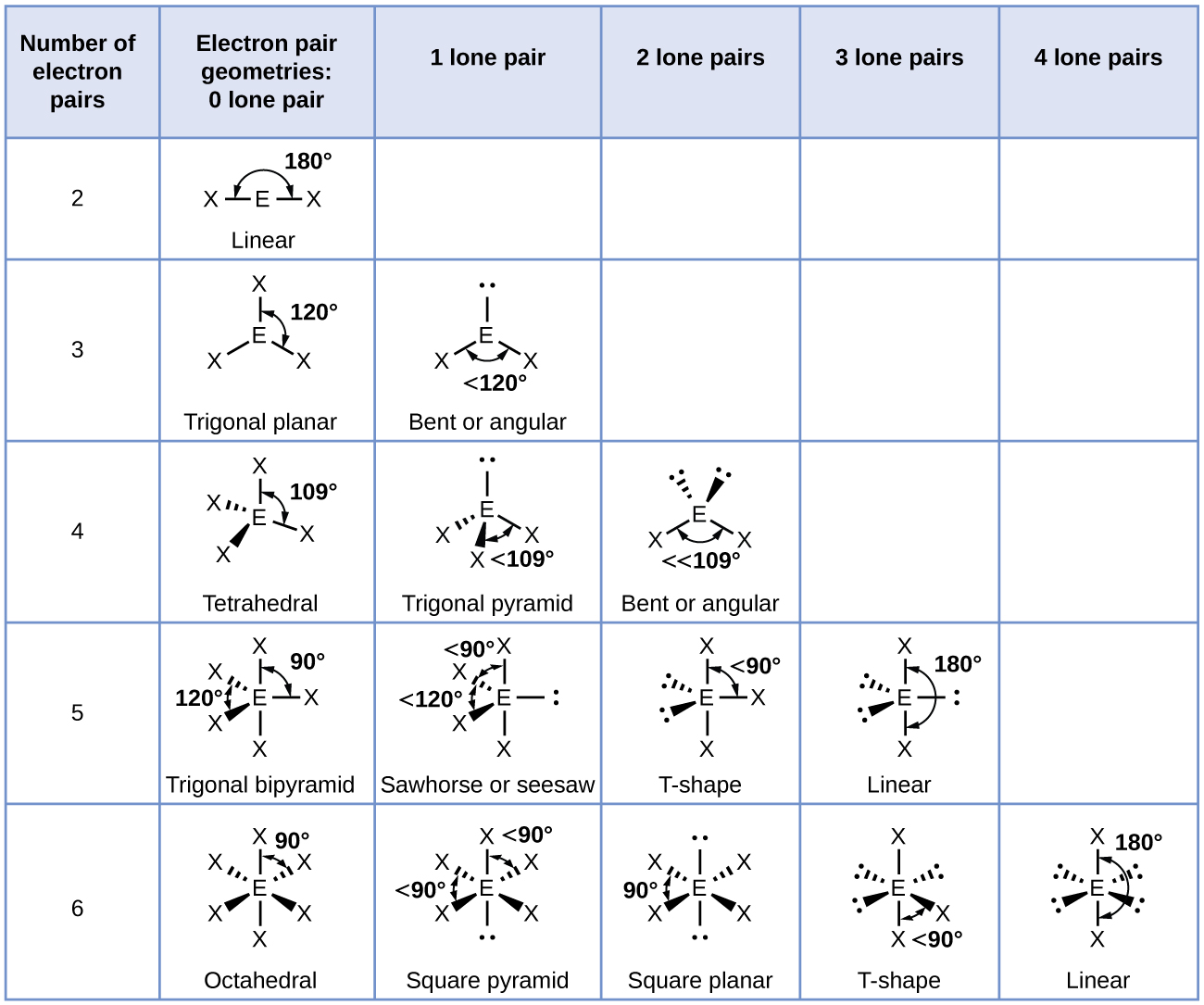 5 2 Molecular Shape Chemistry Libretexts
5 2 Molecular Shape Chemistry Libretexts
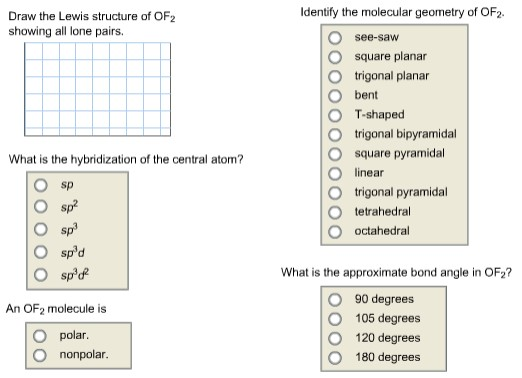 Solved Identify The Molecular Geometry Of Of2 Draw The Le
Solved Identify The Molecular Geometry Of Of2 Draw The Le
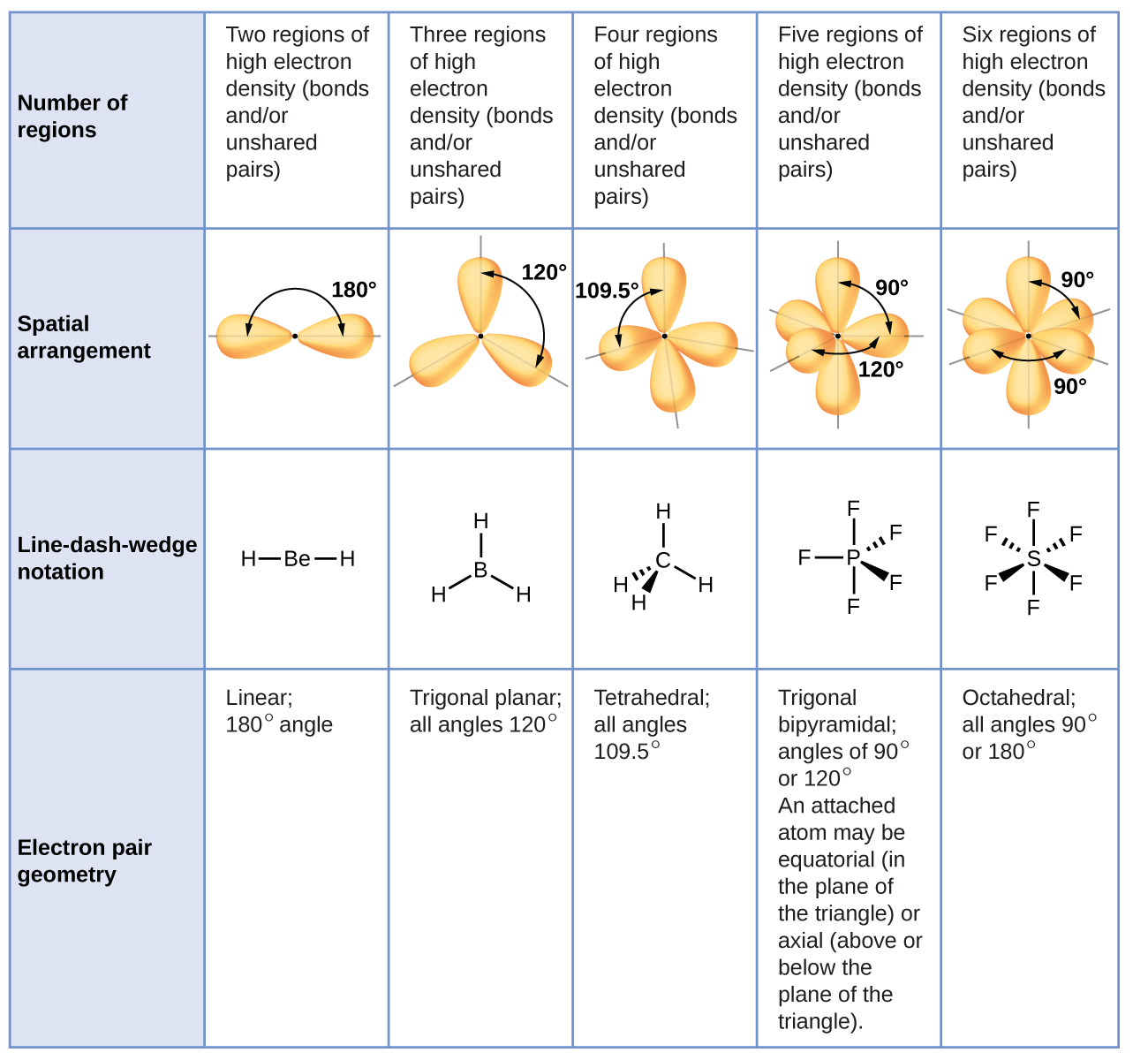 5 2 Molecular Shape Chemistry Libretexts
5 2 Molecular Shape Chemistry Libretexts
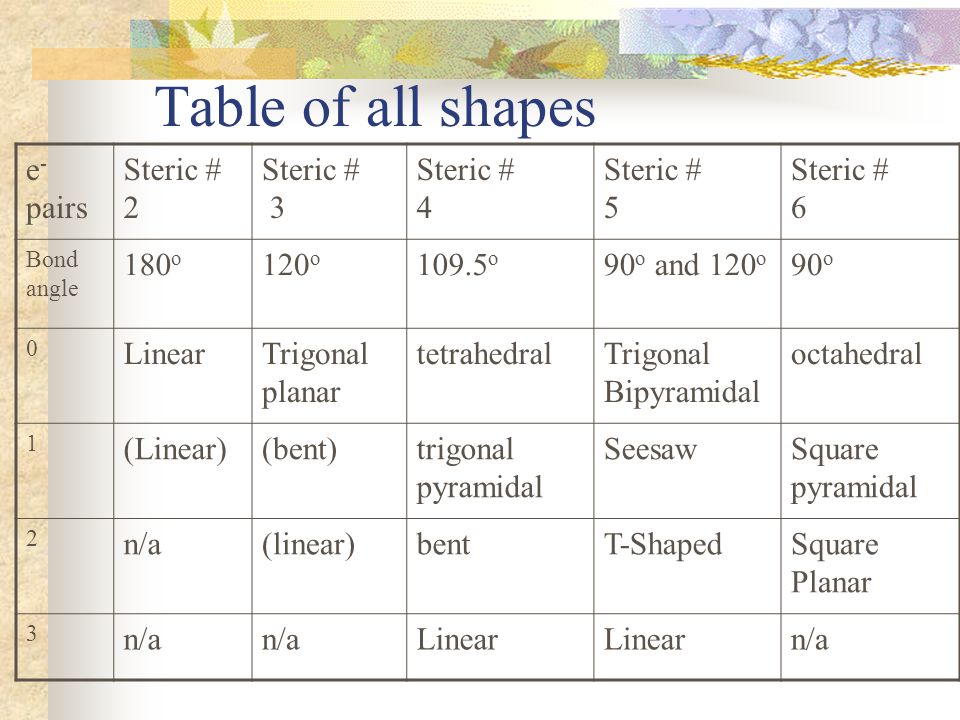 Vsepr Theory Ppt Video Online Download
Vsepr Theory Ppt Video Online Download
Http Www Csus Edu Indiv M Mackj Eit Vespr Pdf
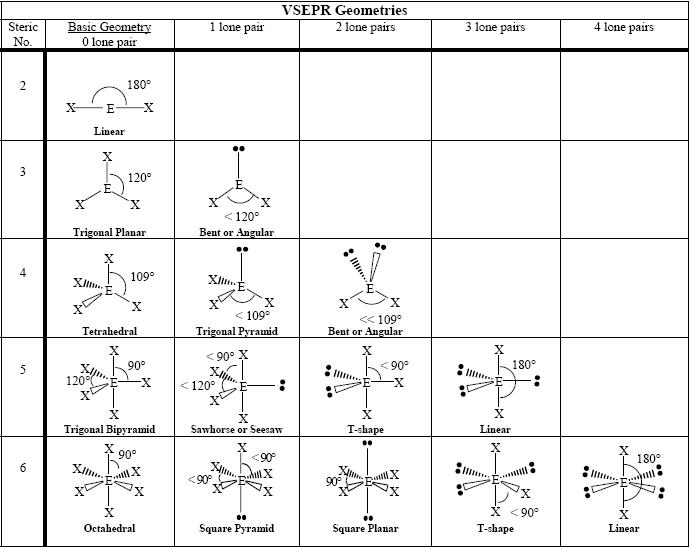 Molecular Geometry Chemistry Socratic
Molecular Geometry Chemistry Socratic
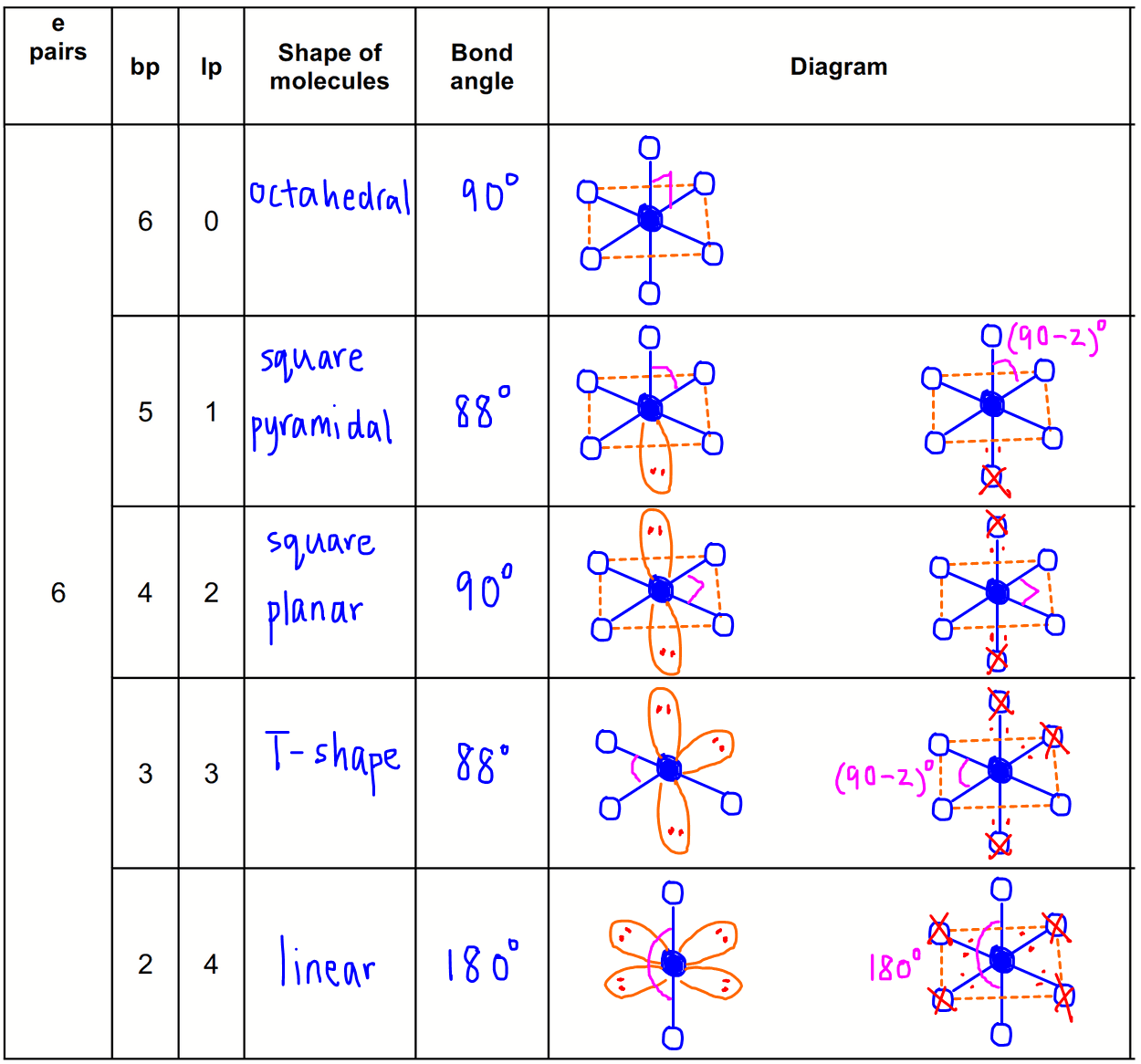 Valence Shell Electron Pair Repulsion Theory
Valence Shell Electron Pair Repulsion Theory
How Do We Determine The Bond Angles For Molecular Geometries That

Posting Komentar
Posting Komentar