Ph Values Of Amino Acids
At a ph of 9 there are equal amounts of the protonated nh 2 and unprotonated forms nh 3. In the ionic forms the amino acids are called aspartate and glutamate.
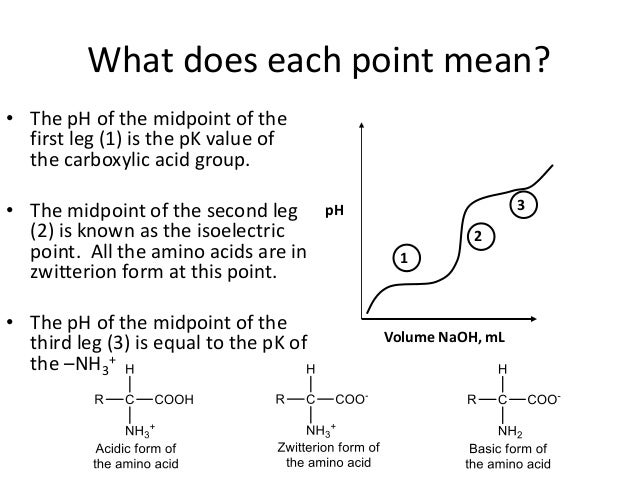
Pka 1 pka 2 and pka 3 are the pka value of the c terminal cooh group a carboxyl group n terminal nh 2 group a ammonium group and the r group if any of the specified amino acid.
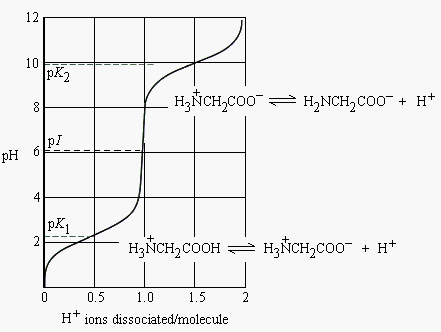
Ph values of amino acids. Each has a carboxylic acid on its side chain that gives it acidic proton donating properties. Explaining why it isn t at ph 7 is quite long winded and almost certainly beyond what you will need for uk a level or its equivalent purposes. Pi is the ph at which the amino acid has a net zero charge.
Thus monoamino monocarboxylic acids exhibit their greatest buffering capacities in the two ph ranges near their two pk values namely ph 2 3 and ph 9 7 figure 3 6. The ratio of the concentrations of the two isomers is independent of ph. These are aspartic acid or aspartate asp and glutamic acid or glutamate glu.
The atom with the lowest pk a will be deprotonated. The pka table of amino acids lists a maximum of three pka values namely pka 1 pka 2 and pka 3. In an aqueous solution at physiological ph all three functional groups on these amino acids will ionize thus giving an overall charge of 1.
The isoelectric point isoelectric ph. For the amino acids with protonated r groups you need to pay attention to their pk a values. The pk a of an nh3 group is typically 9.
The pka values may differ among the reference sources thus. Use the following link to find a list of the pk a values for all the amino acids. Pk a values for amino acids.
If we were to repeat the electrophoresis of these compounds at a ph of 3 80 the aspartic acid would remain at its point of origin and the other amino acids would move toward the cathode. In fact the isoelectric point for many amino acids is about ph 6. If you are interested the problem is discussed at the bottom of this page.
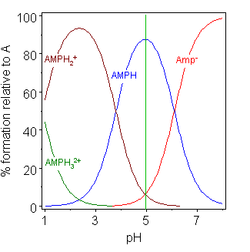
In aqueous solution amino acids exist in two forms as illustrated at the right the molecular form and the zwitterion form in equilibrium with each other. For a simple diprotic amino acid the pi falls halfway between the two pk values. For these amino acids the protonated forms predominate at physiological ph about 7.
Conversely if we look at the amino group nh 2 of a protein it is actually the base part of a conjugate acid base pair in which the acid is the protonated form nh 3. Pka values of polyfunctional amino acids it should be clear that the result of this experiment is critically dependent on the ph of the matrix buffer. The two forms co exist over the ph range pk1 2 to pk2 2 which for glycine is ph 0 12.
Two amino acids have acidic side chains at neutral ph. For acidic amino acids the pi is given by pk1 pk2 and for basic amino acids it s given by pk2 pk3. The exact opposite would happen for protonation of amino acids.
 Amino Acid And Ammonium Noe Factors At Different Ph Values A
Amino Acid And Ammonium Noe Factors At Different Ph Values A
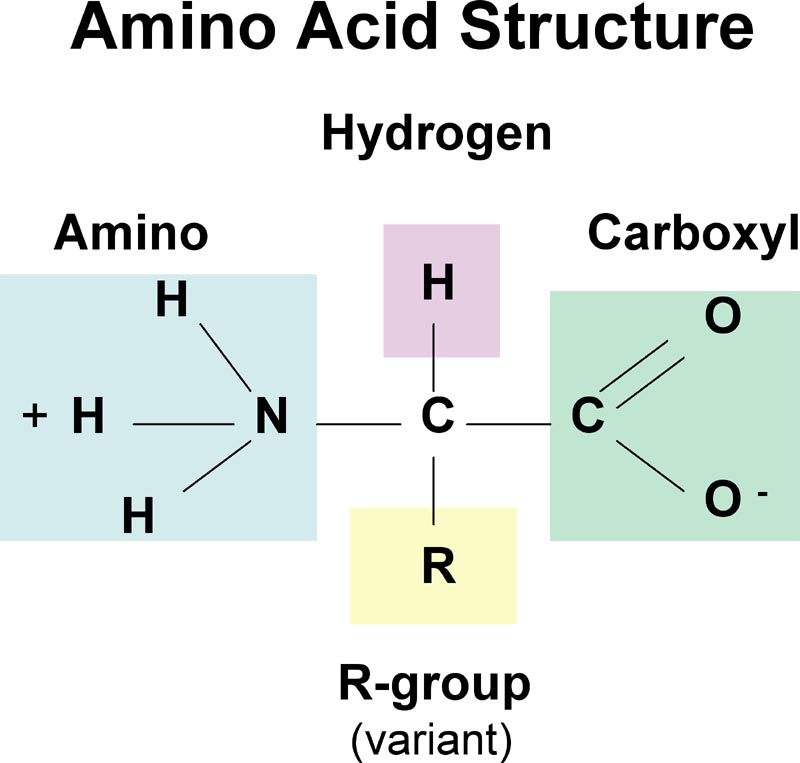 How Does Ph Affect Amino Acid Structure Example
How Does Ph Affect Amino Acid Structure Example
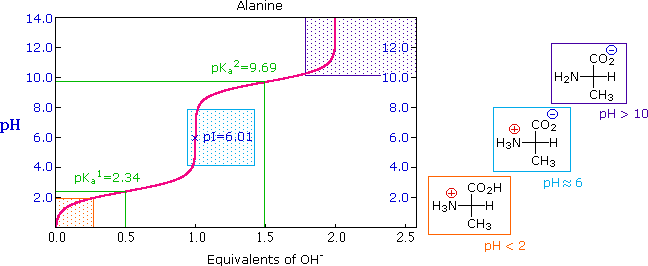 26 4 Amino Acids The Henderson Hasselbalch Equation And
26 4 Amino Acids The Henderson Hasselbalch Equation And
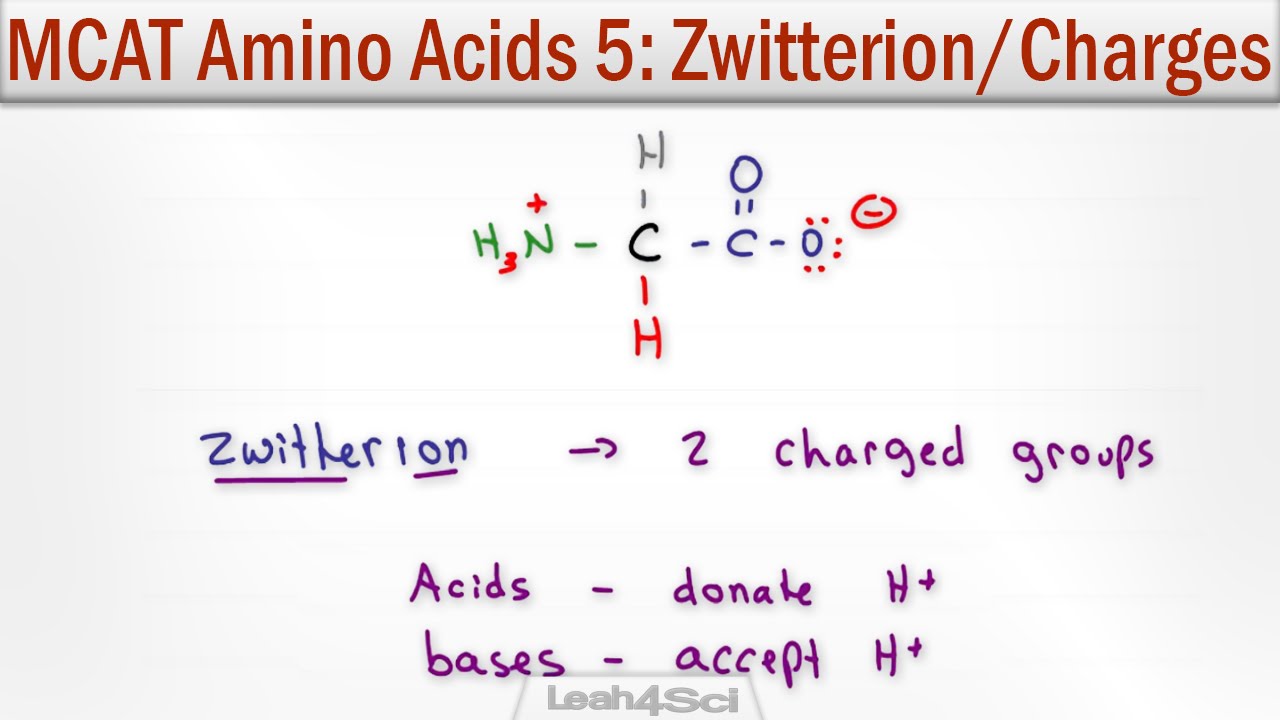 Zwitterion And Amino Acid Charge Given Ph And Pka Youtube
Zwitterion And Amino Acid Charge Given Ph And Pka Youtube
 Zwitterions An Overview Sciencedirect Topics
Zwitterions An Overview Sciencedirect Topics
 Solved For Each Of The Following Amino Acids Draw The St
Solved For Each Of The Following Amino Acids Draw The St
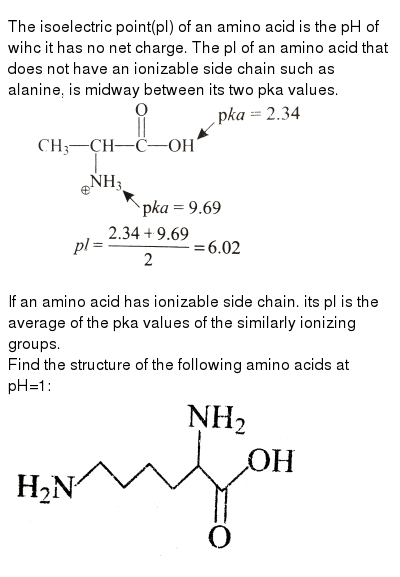 The Isoelectric Point Pl Of An Amino Acid Is The Ph Of Wihc It Ha
The Isoelectric Point Pl Of An Amino Acid Is The Ph Of Wihc It Ha
 Table 1 From The Effect Of Sugar Amino Acid Metal Ion And Nacl
Table 1 From The Effect Of Sugar Amino Acid Metal Ion And Nacl
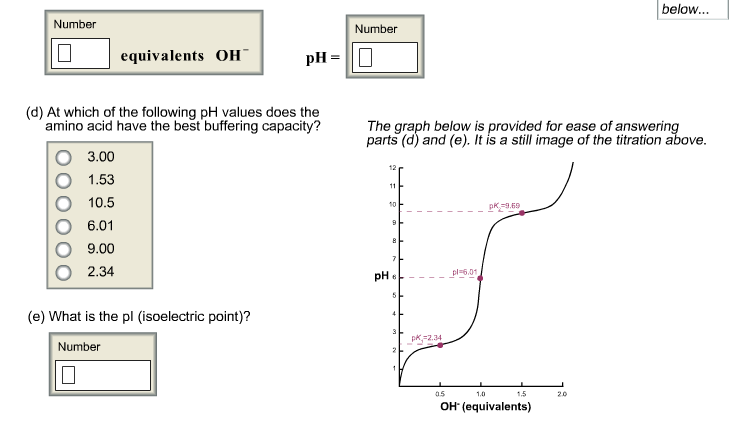 Solved At Which Of The Following Ph Values Does The Amino
Solved At Which Of The Following Ph Values Does The Amino
 Effects Of Different Concentrations Of Three Tested Amino Acids On
Effects Of Different Concentrations Of Three Tested Amino Acids On
Amino Acid Charge In Zwitterions And Isoelectric Point Mcat Tutorial
 Chemistry Of Amino Acids Proteins
Chemistry Of Amino Acids Proteins
 Table 2 From Adsorptive Interaction Of Chiral Amino Acids On B
Table 2 From Adsorptive Interaction Of Chiral Amino Acids On B
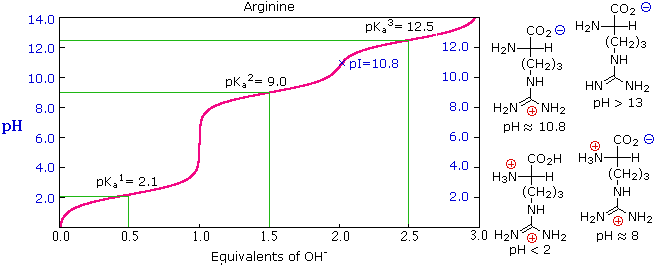 26 4 Amino Acids The Henderson Hasselbalch Equation And
26 4 Amino Acids The Henderson Hasselbalch Equation And
 Acidic And Basic Amino Acids And Total Charge Q T Of Human
Acidic And Basic Amino Acids And Total Charge Q T Of Human
 A Reliable Methodology For Quantitative Extraction Of Fruit And
A Reliable Methodology For Quantitative Extraction Of Fruit And
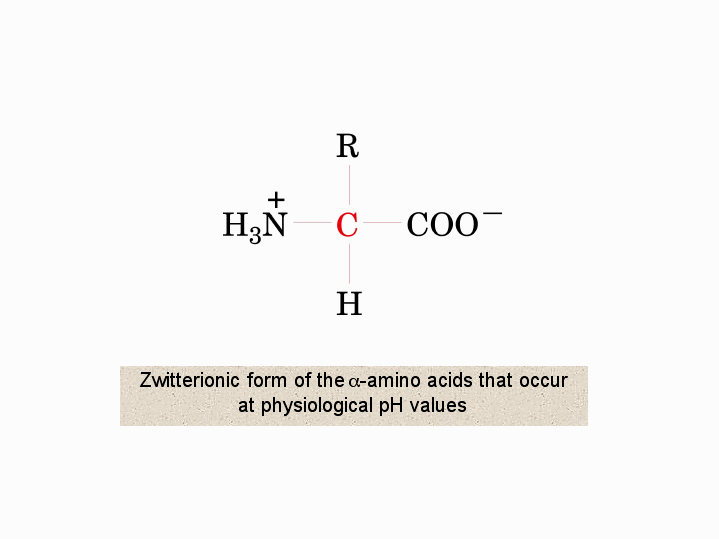 Zwitterionic Form Of The A Amino Acids That Occur At Physiological
Zwitterionic Form Of The A Amino Acids That Occur At Physiological
Amino Acid Charge In Zwitterions And Isoelectric Point Mcat Tutorial
 Study Of Complexes Of Cadmium With Some L Amino Acids And Vitamin
Study Of Complexes Of Cadmium With Some L Amino Acids And Vitamin
 Ph Effects On Amino Acid Structures Youtube
Ph Effects On Amino Acid Structures Youtube
 Lecture 3 Amino Acid Protein Ppt Download
Lecture 3 Amino Acid Protein Ppt Download
 Zwitterions An Overview Sciencedirect Topics
Zwitterions An Overview Sciencedirect Topics
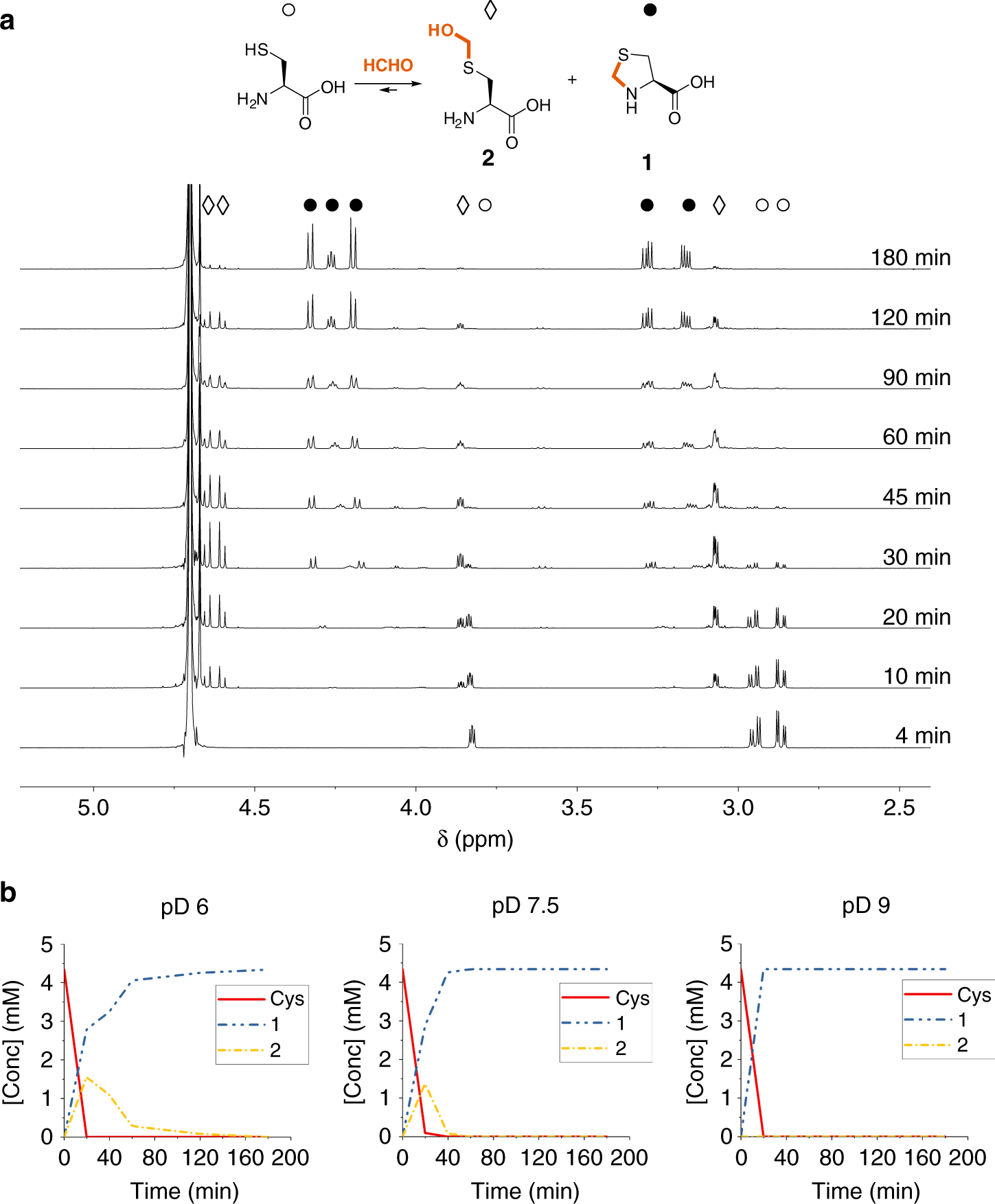 How Formaldehyde Reacts With Amino Acids Communications Chemistry
How Formaldehyde Reacts With Amino Acids Communications Chemistry
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctmzwxk Ldkzlplvlsaqug4gdix9qh7vkd0mjru4yfcudylbi6c Usqp Cau
How Does The R Group Determine The Characteristics Of Amino Acids
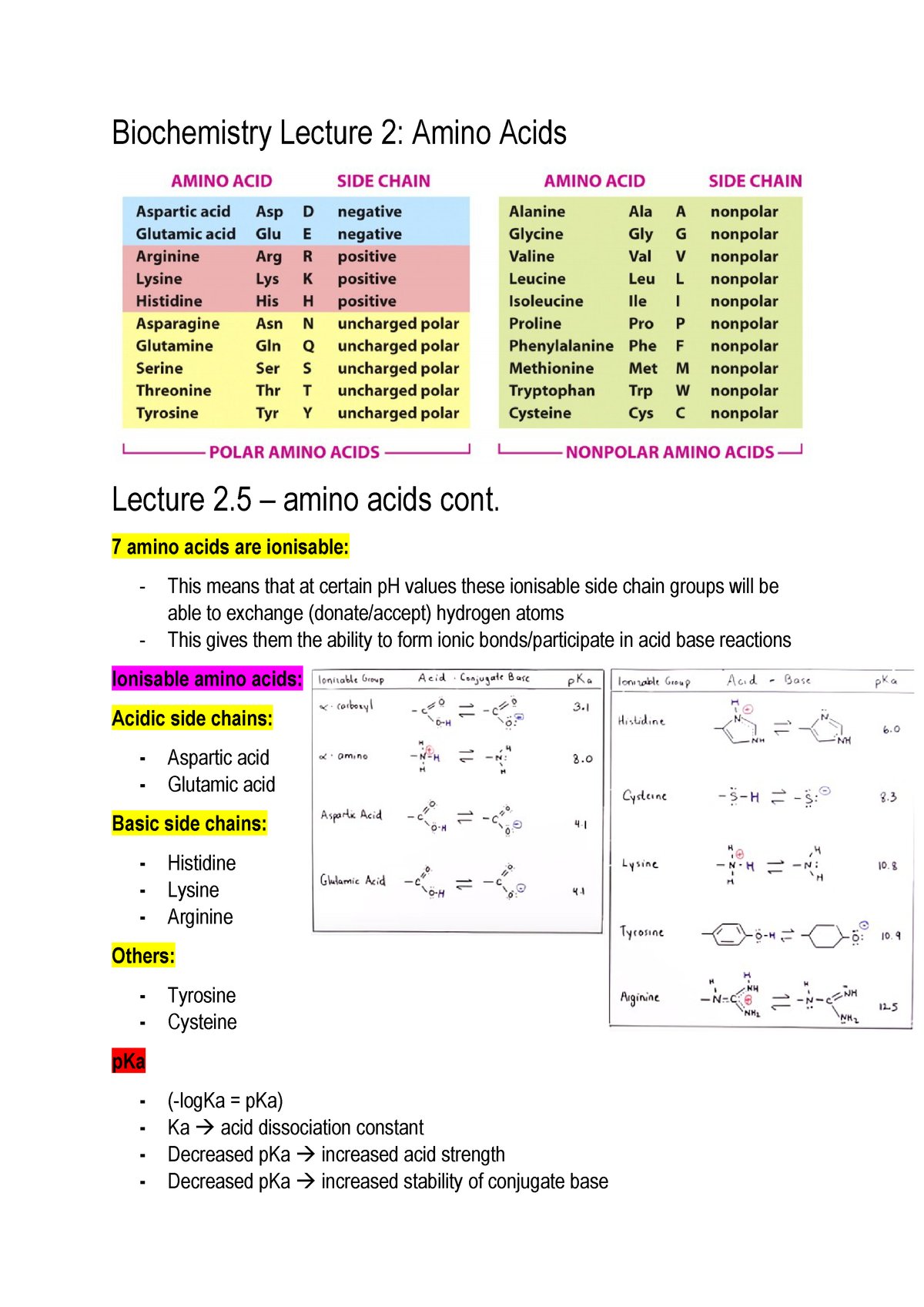 Biochemistry Lecture 2 Unimelb Studocu
Biochemistry Lecture 2 Unimelb Studocu
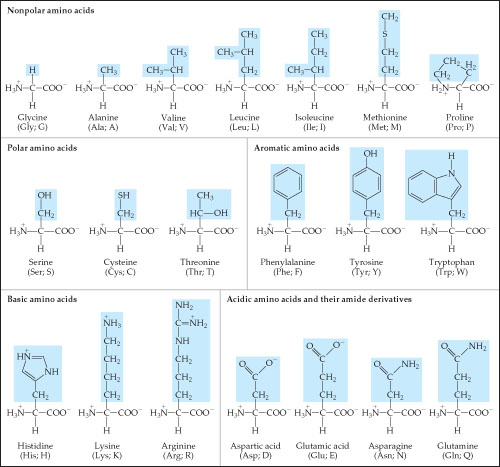 Solved The 20 Amino Acids Found In The Human Body The Ac
Solved The 20 Amino Acids Found In The Human Body The Ac
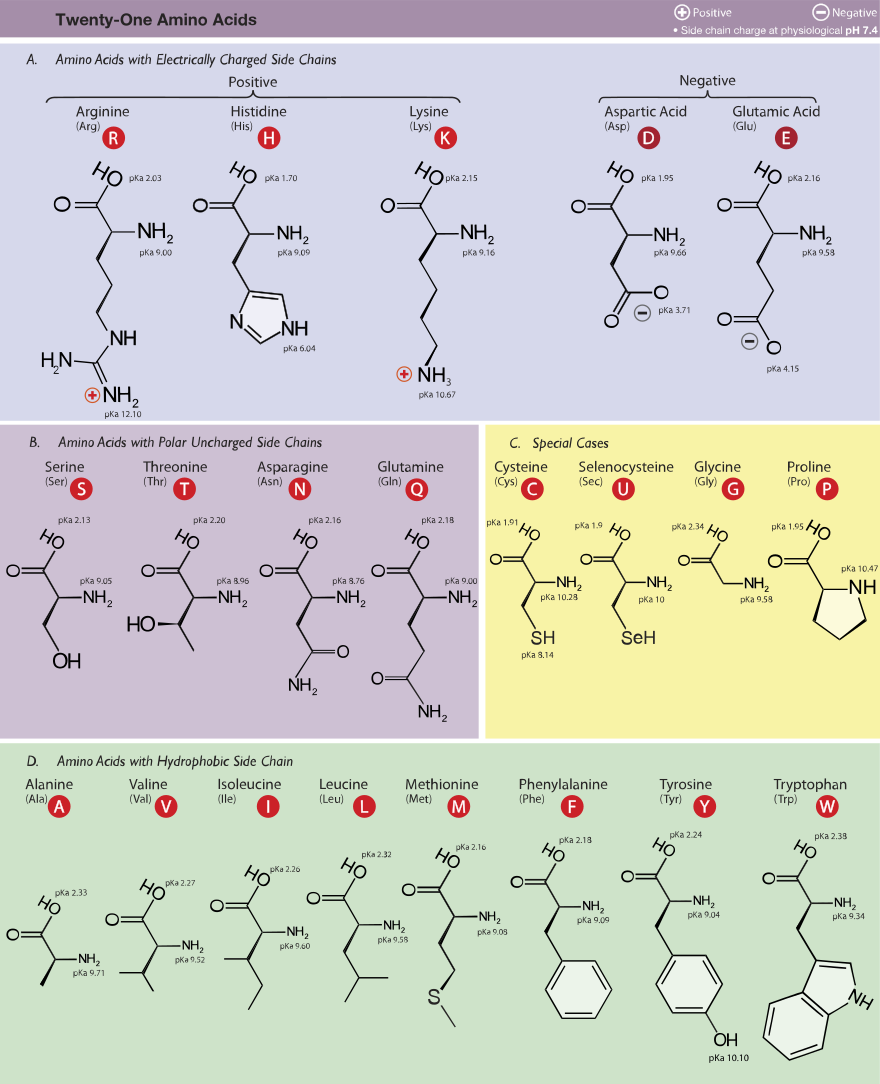



Posting Komentar
Posting Komentar